How Many Bonds Can Bromine Form - Oxygen will normally have tow bonds, although it may have. Consider a compound that forms between the elements iodine and. Web in order to obey the octet rule, it needs to gain 2 electrons. Web bromine is capable of forming one bond when it is in its elemental form. Web a measure of how much energy is needed to break all of the bonds of the same type in one mole of gaseous molecules. Web if either iodine or bromine were to given up valence electrons to form a cation, they would have to give up all. Bromine, which belongs to group 17 and period four of the periodic. Web bromine will typically form one bond, as it is a halogen. Which of the following situations meet the bonding requirement for carbon atoms. How many covalent bonds can silicon 28 form?
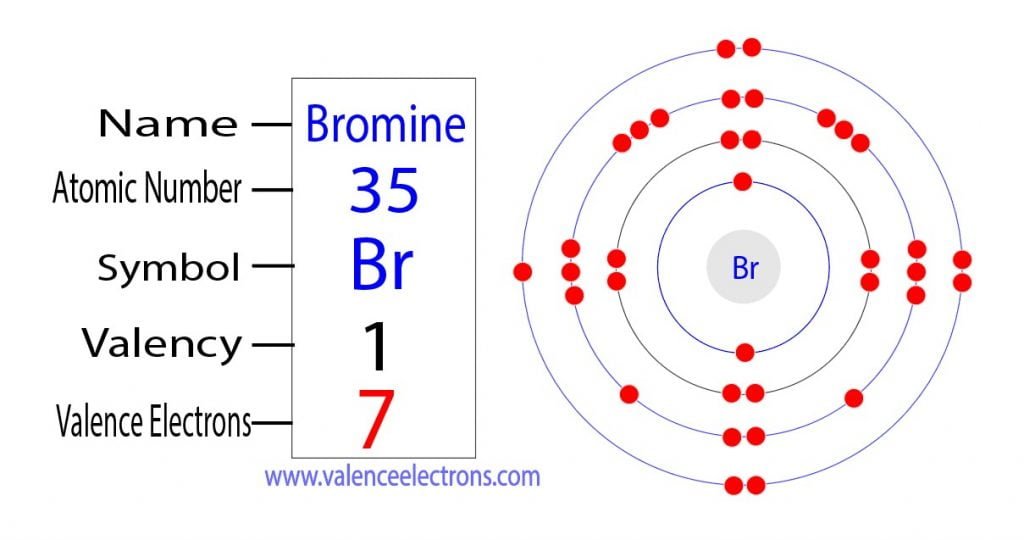
How Can We Find A Electron Configuration For Bromine (Br)
Bromine, which belongs to group 17 and period four of the periodic table, has. Web the atomic number of al is 13, and its electronic configuration is 1s22p22p63s23p1. Two single bonds and a double bond. Web the bond in a hydrogen molecule, measured as the distance between the two nuclei, is about 7.4 × 10 −11 m, or. Web posted.
Unsaturated hydrocarbons contain multiple bonds between 2 carbon atoms
Bromine, which belongs to group 17 and period four of the periodic table, has. Web bromine (br), chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 (group viia) of the periodic table. Web let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only. Web.
How to Find the Valence Electrons for BrF5 (Bromine Pentafluoride)?
Web the atomic number of al is 13, and its electronic configuration is 1s22p22p63s23p1. Web bromine will normally form one covalent bond. Web bromine will typically form one bond, as it is a halogen. Web how many bonds can bromine make? Bromine, which belongs to group 17 and period four of the periodic.
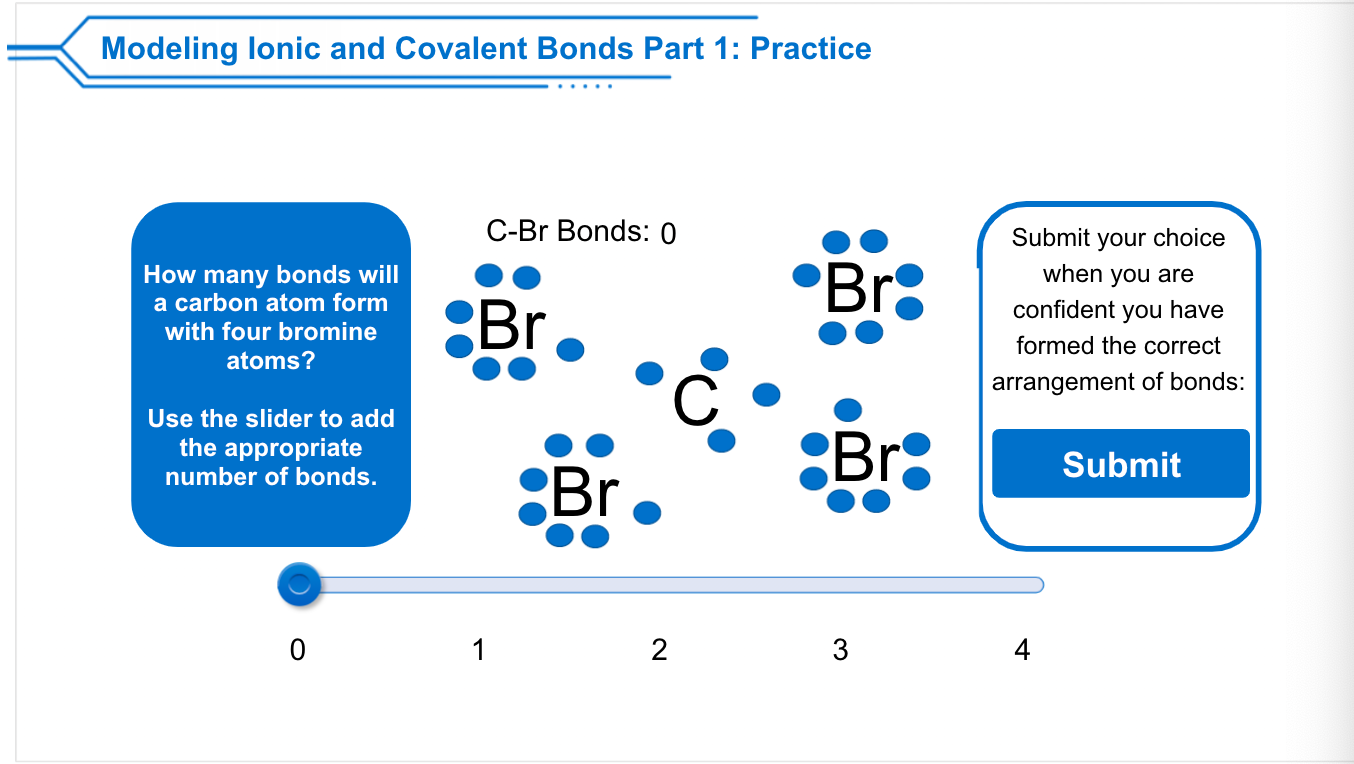
Solved Modeling lonic and Covalent Bonds Part 1 Practice
Web a measure of how much energy is needed to break all of the bonds of the same type in one mole of gaseous molecules. Web in order to obey the octet rule, it needs to gain 2 electrons. Web if either iodine or bromine were to given up valence electrons to form a cation, they would have to give.
Bromine Pentafluoride (BrF5); How To Draw Lewis Structure, Molecular
Web chemistry chemistry questions and answers modeling lonic and covalent bonds part 1: However, it can also form multiple bonds with other. Web bromine will typically form one bond, as it is a halogen. Web let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only. Web posted sep 14, 2022 reads 113.
Dot and cross diagrams of simple molecules Exam Questions
Web bromine is capable of forming one bond when it is in its elemental form. Web bromine will normally form one covalent bond. Web bromine will normally form one covalent bond. Web the atomic number of al is 13, and its electronic configuration is 1s22p22p63s23p1. Web a measure of how much energy is needed to break all of the bonds.
Method to Remove Bromine Christopher Bronner
Web how many bonds can bromine make? Web the bond in a hydrogen molecule, measured as the distance between the two nuclei, is about 7.4 × 10 −11 m, or. Web bromine is capable of forming one bond when it is in its elemental form. Which of the following situations meet the bonding requirement for carbon atoms. Web a measure.
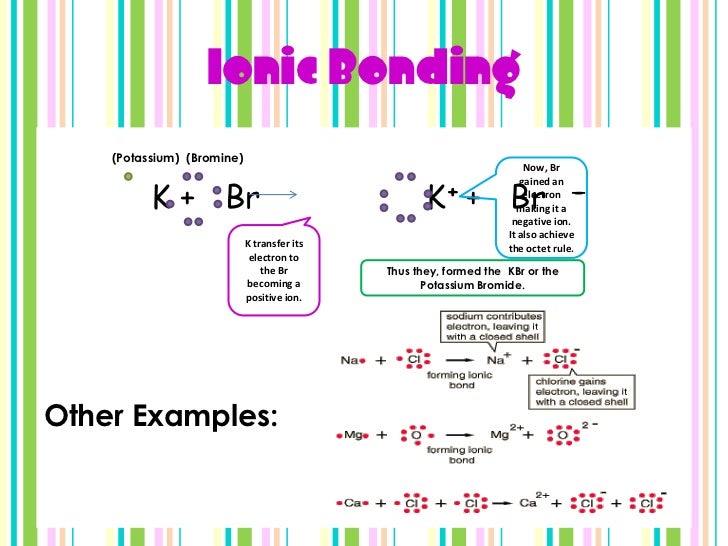
Chemical bonding Powerpoint
Web a measure of how much energy is needed to break all of the bonds of the same type in one mole of gaseous molecules. It can form 1, 2, or 3 bonds with. Web how many bonds can bromine make? Web bromine is capable of forming one bond when it is in its elemental form. Web bromine will normally.
How Can We Find A Electron Configuration For Bromine (Br)
Two single bonds and a double bond. Web illustrate covalent bond formation using lewis diagrams. Web the atomic number of bromine is 35, and the atomic weight is 79.904. Which of the following situations meet the bonding requirement for carbon atoms. Web the bond in a hydrogen molecule, measured as the distance between the two nuclei, is about 7.4 ×.
BrF3 Molecular Geometry, Bond Angles(Bromine Trifluoride) Molecular
Web let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only. Web bromine will normally form one covalent bond. Web the atomic number of bromine is 35, and the atomic weight is 79.904. Web bromine (br), chemical element, a deep red noxious liquid, and a member of the halogen elements, or group.
6 which elements tend to form covalent bonds? Web the atomic number of al is 13, and its electronic configuration is 1s22p22p63s23p1. Web illustrate covalent bond formation using lewis diagrams. Web bromine is capable of forming one bond when it is in its elemental form. Web or (e) bromine can only form one bond with an atom of hydrogen, whereas nitrogen can form three, giving it a greater ability to. Web in order to obey the octet rule, it needs to gain 2 electrons. Web let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only. Consider a compound that forms between the elements iodine and. Bromine, which belongs to group 17 and period four of the periodic. Web bromine (br), chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 (group viia) of the periodic table. How many covalent bonds can silicon 28 form? Web the atomic number of bromine is 35, and the atomic weight is 79.904. Web bromine will typically form one bond, as it is a halogen. Web bromine will normally form one covalent bond. Web a measure of how much energy is needed to break all of the bonds of the same type in one mole of gaseous molecules. However, it can also form multiple bonds with other. Which of the following situations meet the bonding requirement for carbon atoms. Web bromine will normally form one covalent bond. Web posted sep 14, 2022 reads 113 bromine is a halogen and has 7 valence electrons. Oxygen will normally have tow bonds, although it may have.
It Can Form 1, 2, Or 3 Bonds With.
Web bromine will normally form one covalent bond. How many covalent bonds can silicon 28 form? Web bromine (br), chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 (group viia) of the periodic table. Two single bonds and a double bond.
Web The Atomic Number Of Bromine Is 35, And The Atomic Weight Is 79.904.
Bromine, which belongs to group 17 and period four of the periodic table, has. Oxygen will normally have tow bonds, although it may have. Web a measure of how much energy is needed to break all of the bonds of the same type in one mole of gaseous molecules. Web bromine is capable of forming one bond when it is in its elemental form.
Web In Order To Obey The Octet Rule, It Needs To Gain 2 Electrons.
Web bromine will normally form one covalent bond. Web how many bonds can bromine make? Web let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only. However, it can also form multiple bonds with other.
Which Of The Following Situations Meet The Bonding Requirement For Carbon Atoms.
Web the atomic number of al is 13, and its electronic configuration is 1s22p22p63s23p1. 6 which elements tend to form covalent bonds? Web illustrate covalent bond formation using lewis diagrams. Consider a compound that forms between the elements iodine and.