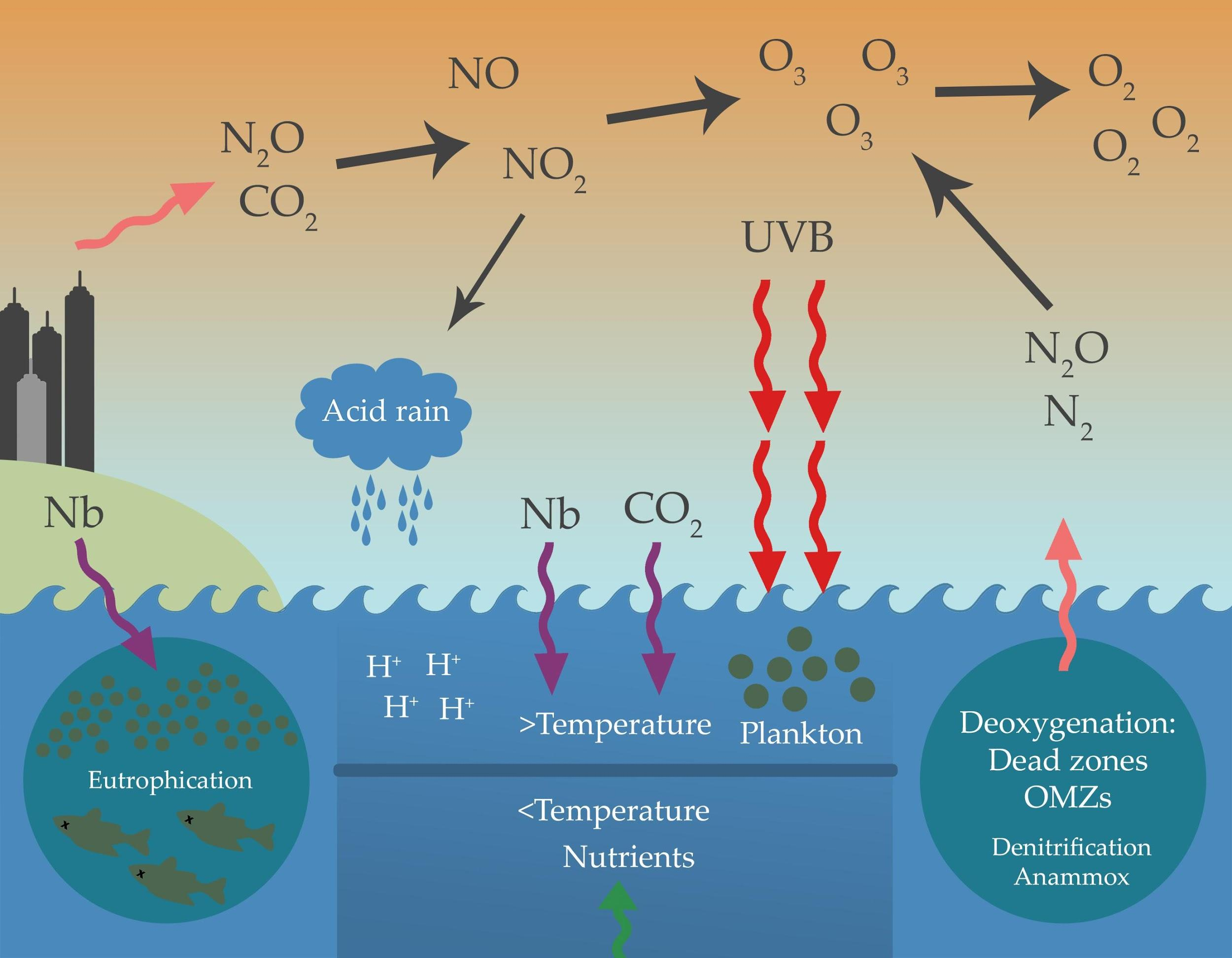
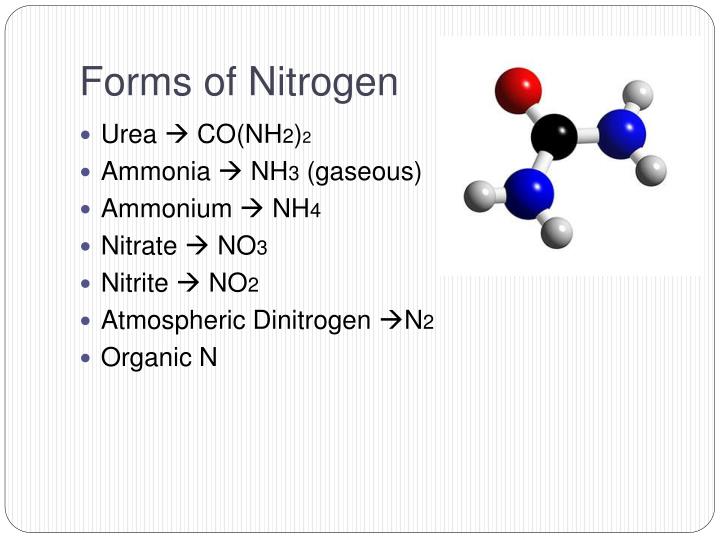
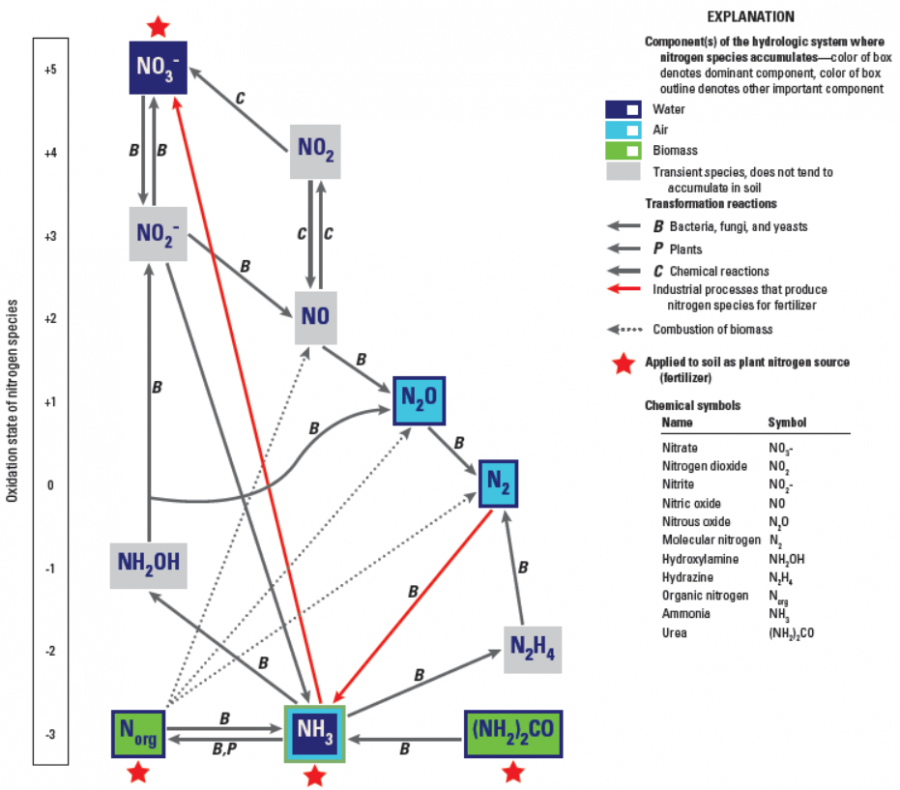
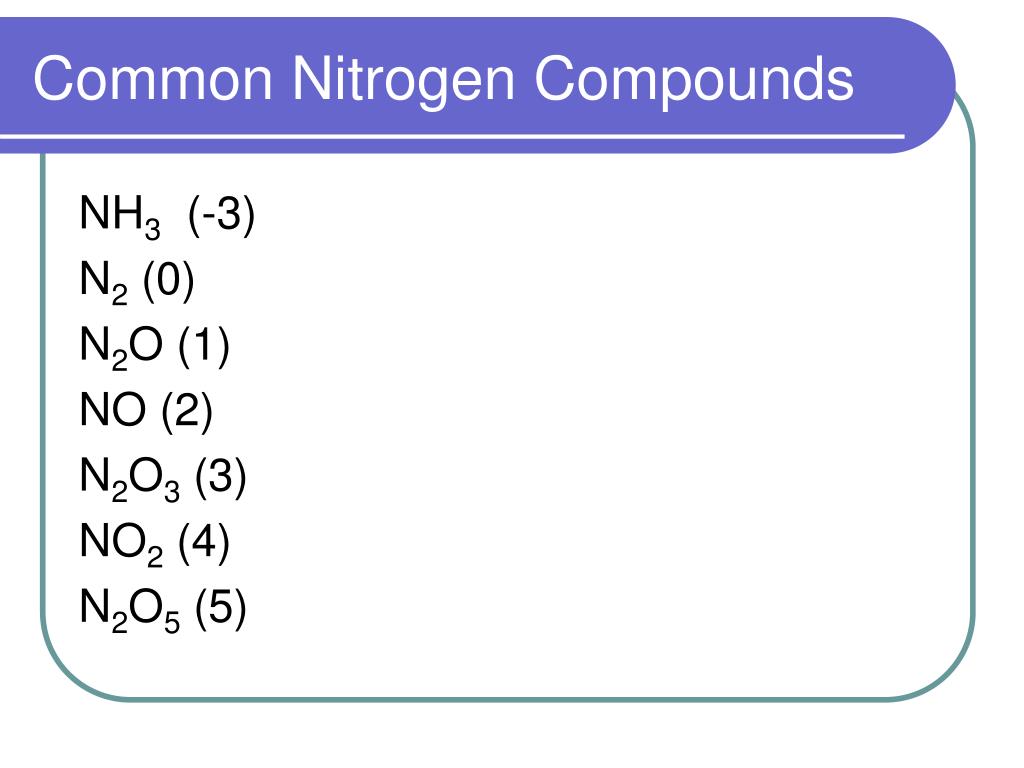
Explain How An Anion Of Nitrogen Forms - Web for groups 1, 2, 13, and 14, the elements have one to four valence electrons as neutral atoms, and they will usually give away these valence electrons to. An atom gains or loses electrons to achieve a stable electron configuration. When animals eat the plants, they acquire usable nitrogen compounds. Web when formula ionic compounds, nitrogen will become an an eye on to understand how it becomes an anti on, let's look at its orbital. Web the ammonium ion (see figure below) consists of one nitrogen atom and four hydrogen atoms. Web video answer:my coaching for months. Web an atom of nitrogen contains 7 protons and 7 electrons, 5 of which can be classified as valence electrons, as determined either by. Okay, so we're asked to explain how anna of majin forms. Web solved:explain how an anion of nitrogen forms. Web when formula ionic compounds, nitrogen will become an an eye on to understand how it becomes an anti on, let's look at its orbital.
With the help of a labeled diagram show A The nitrogen class 12 biology
Web explain how an anion of nitrogen forms a nitrogen atom must gain three electrons to have the same number of electrons as an. Okay, so we're asked to explain how anna of majin forms. Nonmetals form negative ions (anions). Web video answer:my coaching for months. Web since the ions have charges that are equal in magnitude (3, but different.
What is Nitrogen? Definition, Formula, Cycle, Fixation
An atom gains or loses electrons to achieve a stable electron configuration. Web solved:explain how an anion of nitrogen forms. When animals eat the plants, they acquire usable nitrogen compounds. Web in this process, the carbon flux is obtained by evaporation of graphite, typically by an electron beam. Web how do positive ions and negative ions form?
PPT Nitrogen Cycle PowerPoint Presentation ID5576516
Nonmetals form negative ions (anions). Okay, so we're asked to explain how anna of majin forms. You add three electrons to nitrogen's outer most shell. Web explain how an anion of nitrogen forms? Explain how iron can form both.
Nitrogen Definition, Symbol, Uses, Properties, Atomic Number, & Facts
Web solved:explain how an anion of nitrogen forms. Web in this process, the carbon flux is obtained by evaporation of graphite, typically by an electron beam. Web nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. Okay, so we're asked to explain how anna of majin forms. An atom gains or loses electrons to achieve.
Describe the flow of matter in the nitrogen cycle Middle School Life
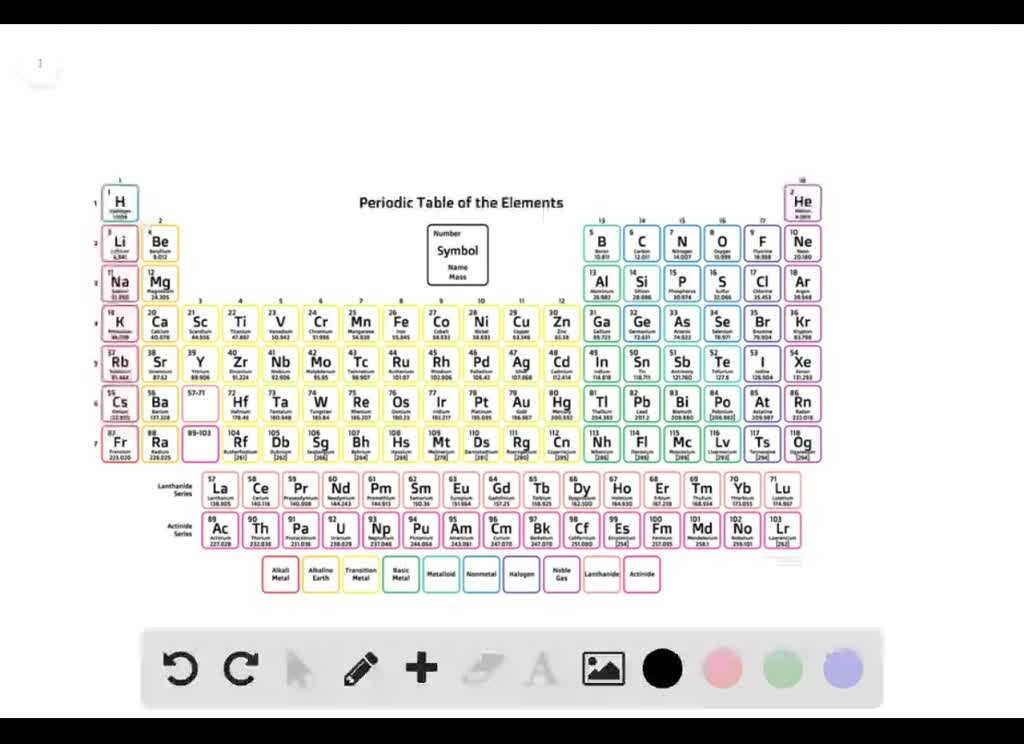
Nitrogen is a common limiting nutrient. Web nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. Explain how iron can form both. An atom gains or loses electrons to achieve a stable electron configuration. Nonmetals form negative ions (anions).
PPT Soil Microbiology PowerPoint Presentation ID966927
Leave out all charges and all subscripts that are 1. Web nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. An atom gains or loses electrons to achieve a stable electron configuration. Web since the ions have charges that are equal in magnitude (3, but different signs), 1:1 is the lowest ratio of ions that..
How to find Valency? What are valence electrons? Teachoo
Web explain how an anion of nitrogen forms? When animals eat the plants, they acquire usable nitrogen compounds. It is a colorless, odorless, tasteless gas that is. So let's look up, nitrogen. Web an atom of nitrogen contains 7 protons and 7 electrons, 5 of which can be classified as valence electrons, as determined either by.
What Are the 4 Steps of Nitrogen Cycle? Earth How
An atom gains or loses electrons to achieve a stable electron configuration. Web video answer:my coaching for months. Leave out all charges and all subscripts that are 1. Web when formula ionic compounds, nitrogen will become an an eye on to understand how it becomes an anti on, let's look at its orbital. Like carbon, nitrogen tends to form ionic.
PPT Nitrogen in All Its Forms PowerPoint Presentation, free download
Web nitrogen does not share the proclivity of carbon for catenation. Web an atom of nitrogen contains 7 protons and 7 electrons, 5 of which can be classified as valence electrons, as determined either by. Explain how iron can form both. Web solved:explain how an anion of nitrogen forms. Together, they comprise a single.
SOLVEDExplain how an anion of nitrogen forms.
Nonmetals form negative ions (anions). Web nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. So let's look up, nitrogen. It is a colorless, odorless, tasteless gas that is. Nitrogen is a common limiting nutrient.
Nitrogen is a common limiting nutrient. Web in this process, the carbon flux is obtained by evaporation of graphite, typically by an electron beam. Web nitrogen’s position in the periodic table reveals that it is a nonmetal. So we know nitrogen is a non metal and non metals for negative ions. Web explain how an anion of nitrogen forms a nitrogen atom must gain three electrons to have the same number of electrons as an. Web when formula ionic compounds, nitrogen will become an an eye on to understand how it becomes an anti on, let's look at its orbital. Explain how iron can form both. Web nitrogen, nonmetallic element of group 15 [va] of the periodic table. Web explain how an anion of nitrogen forms? So let's look up, nitrogen. Web solved:explain how an anion of nitrogen forms. Web an atom of nitrogen contains 7 protons and 7 electrons, 5 of which can be classified as valence electrons, as determined either by. Web in addition to n 2 and nh 3, nitrogen exists in many different forms, including both inorganic (e.g., ammonia, nitrate) and. Web nitrogen does not share the proclivity of carbon for catenation. Nonmetals form negative ions (anions). You add three electrons to nitrogen's outer most shell. Web for groups 1, 2, 13, and 14, the elements have one to four valence electrons as neutral atoms, and they will usually give away these valence electrons to. Web since the ions have charges that are equal in magnitude (3, but different signs), 1:1 is the lowest ratio of ions that. Like carbon, nitrogen tends to form ionic or metallic compounds with metals. When animals eat the plants, they acquire usable nitrogen compounds.
Like Carbon, Nitrogen Tends To Form Ionic Or Metallic Compounds With Metals.
Explain how iron can form both. Web how do positive ions and negative ions form? Web for groups 1, 2, 13, and 14, the elements have one to four valence electrons as neutral atoms, and they will usually give away these valence electrons to. Nonmetals form negative ions (anions).
So Let's Look Up, Nitrogen.
It is a colorless, odorless, tasteless gas that is. Web nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. Nitrogen is a common limiting nutrient. Web in addition to n 2 and nh 3, nitrogen exists in many different forms, including both inorganic (e.g., ammonia, nitrate) and.
Web The Ammonium Ion (See Figure Below) Consists Of One Nitrogen Atom And Four Hydrogen Atoms.
Web since the ions have charges that are equal in magnitude (3, but different signs), 1:1 is the lowest ratio of ions that. Web nitrogen does not share the proclivity of carbon for catenation. Nonmetals form negative ions (anions). An atom gains or loses electrons to achieve a stable electron configuration.
Web Nitrogen’s Position In The Periodic Table Reveals That It Is A Nonmetal.
Web an atom of nitrogen contains 7 protons and 7 electrons, 5 of which can be classified as valence electrons, as determined either by. Together, they comprise a single. Web explain how an anion of nitrogen forms a nitrogen atom must gain three electrons to have the same number of electrons as an. Web solved:explain how an anion of nitrogen forms.