Enter The Ions Formed When Nh4 2S Dissolves In Water - (a) cobalt (ii) chloride (b) magnesium bromide (c) ammonium. You'll get a detailed solution from. Web the salt ammonium sulfate dissolves in water according to the reaction: Web when the ionic compound #nh_4cl# dissolves in water, it breaks into one ammonium, #nh_4^+# and one. Web which of the following is formed when (nh4)2s04 dissolves in water? What is the name for the compound (nh 4 ) 2 s?. Web b) the compound ammonium sulfate, (nh 4) 2 so 4 is soluble in water. This problem has been solved! Web show how many ions are made when each dissolves in water. Write the net ionic equation for the.
How to Write the Net Ionic Equation for (NH4)2S + FeCl3 = Fe2S3 + NH4Cl
Web which set of ions is formed when (nh4)2so4 dissolves in water? Web when the ionic compound #nh_4cl# dissolves in water, it breaks into one ammonium, #nh_4^+# and one. This problem has been solved! Web since hcl is a strong acid, ka is immeasurably large and kb ≈ 0 (chloride ions don’t undergo appreciable hydrolysis). Web enter the ions formed.
Equation for (NH4)2SO4 + H2O (Ammonium sulfate + Water) YouTube
Web chemistry for the compound \mathrm {li}_2 \mathrm {o} li2o, show that the charges on the ions add up to zero. Web when the ionic compound #nh_4cl# dissolves in water, it breaks into one ammonium, #nh_4^+# and one. Web 4 students found this answer helpful. Enter the ions formed when (nh4)2s dissolves in water. Web since hcl is a strong.
Solved The following chemical reaction takes place in
(a) cobalt (ii) chloride (b) magnesium bromide (c) ammonium. The ions formed are nh4 (+) and. Web chemistry chemistry questions and answers enter the ions formed when (nh4)2 s dissolves in water. Web an electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Web 4 students found this answer helpful.
How to Write the Net Ionic Equation for (NH4)2SO4 + Ba(NO3)2 = NH4NO3
Web show how many ions are made when each dissolves in water. (a) cobalt (ii) chloride (b) magnesium bromide (c) ammonium. Web chemistry chemistry questions and answers enter the ions formed when (nh4)2 s dissolves in water. Web since hcl is a strong acid, ka is immeasurably large and kb ≈ 0 (chloride ions don’t undergo appreciable hydrolysis). Web an.
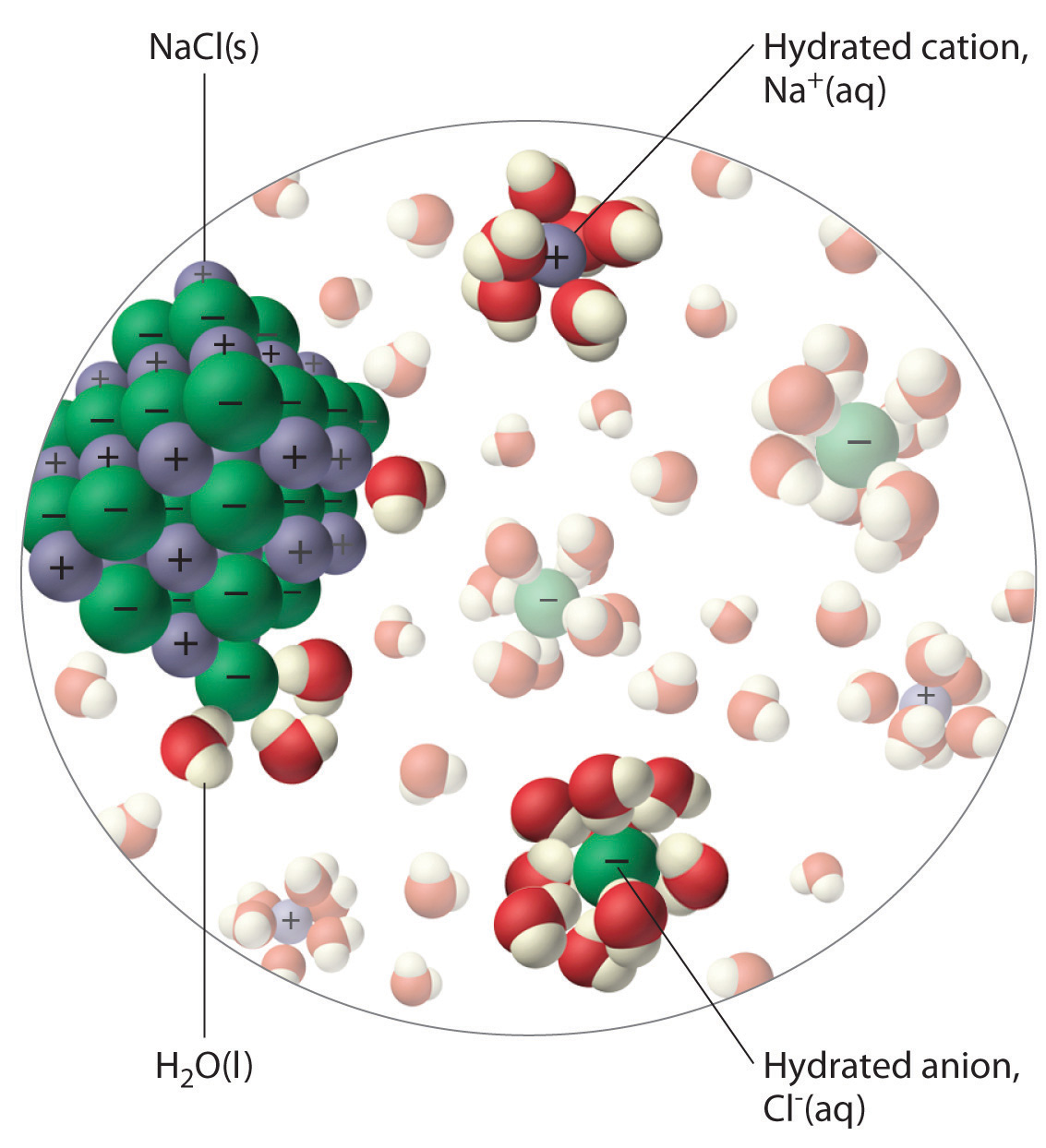
What is it called when sodium and chloride ions separate when dissolved
Web an electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Web b) the compound ammonium sulfate, (nh 4) 2 so 4 is soluble in water. Web 4 students found this answer helpful. Web which set of ions is formed when (nh4)2so4 dissolves in water? You'll get a detailed solution from.
Chemistry Archive February 11, 2018
Weak electrolytes, such as hgcl 2, conduct badly because they produce few ions when dissolved (low concentration of ions) and exist mainly in the form of molecules. Enter the ions formed when (nh4)2s dissolves in water. Web about this tutor ›. Web which of the following is formed when (nh4)2s04 dissolves in water? One important aspect about ionic compounds.
Chapter 14.1 Compounds in Aqueous Solutions
One important aspect about ionic compounds. Web when the ionic compound #nh_4cl# dissolves in water, it breaks into one ammonium, #nh_4^+# and one. Web since hcl is a strong acid, ka is immeasurably large and kb ≈ 0 (chloride ions don’t undergo appreciable hydrolysis). Web chemistry chemistry questions and answers enter the ions formed when (nh4)2 s dissolves in water..
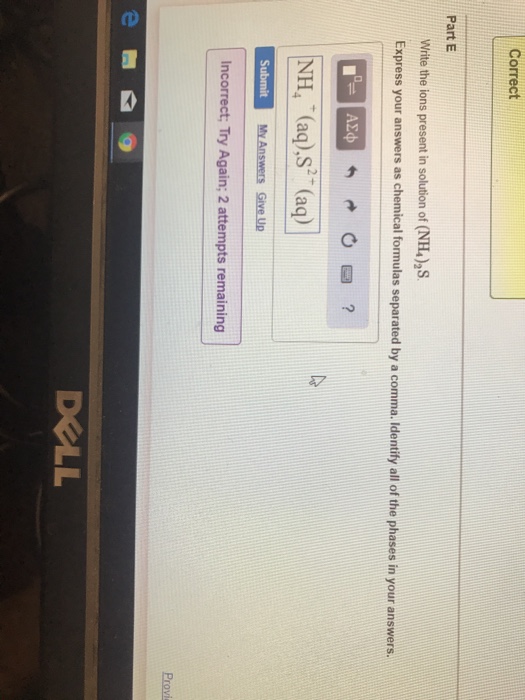
Solved Correct Part E Write the ions present in solution of
Web the salt ammonium sulfate dissolves in water according to the reaction: One important aspect about ionic compounds. Web an electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Web 4 students found this answer helpful. Write the net ionic equation for the.
NH4+ Lewis Structure How to Draw the Dot Structure for NH4+ (Ammonium
Web enter the ions formed when (nh4)2s dissolves in water. Thus, the nh 4+ will. Weak electrolytes, such as hgcl 2, conduct badly because they produce few ions when dissolved (low concentration of ions) and exist mainly in the form of molecules. Web about this tutor ›. Write the net ionic equation for the.
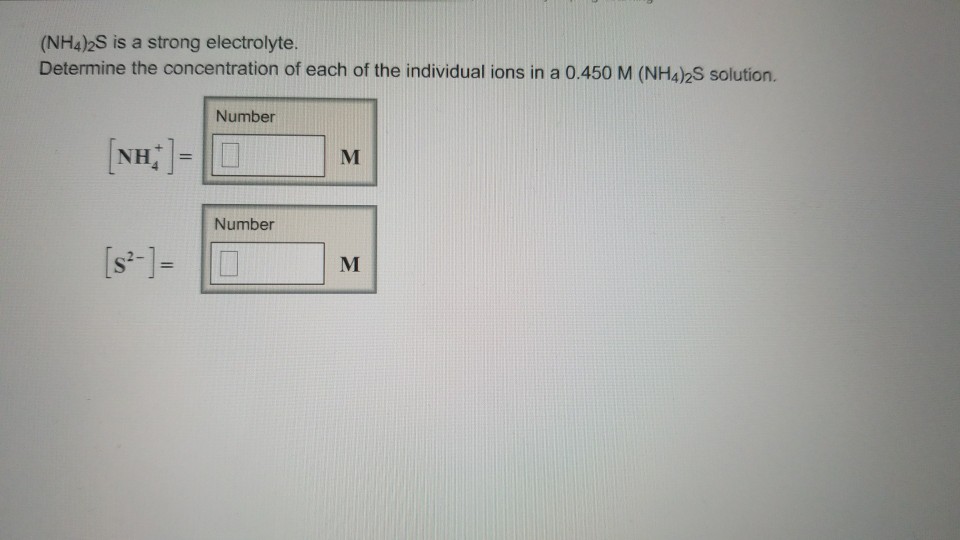
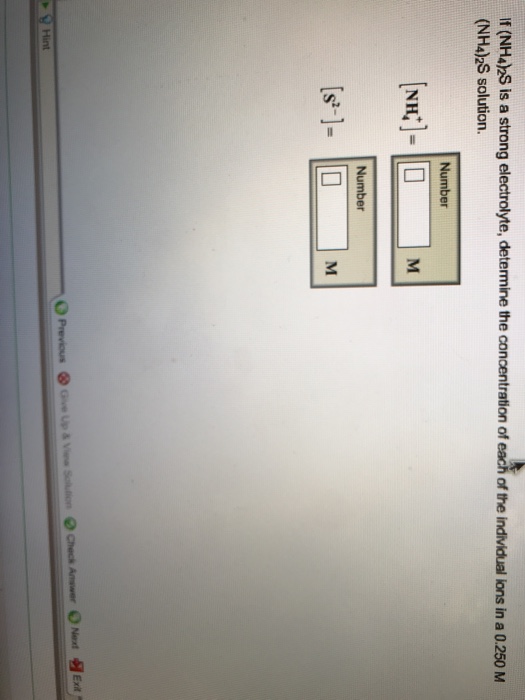
Solved If (NH4)2S is a strong electrolyte, determine the
Web science chemistry chemistry questions and answers enter the ions formed when (nh4)2 s dissolves in water. Thus, the nh 4+ will. Web enter the ions formed when (nh4)2s dissolves in water. Weak electrolytes, such as hgcl 2, conduct badly because they produce few ions when dissolved (low concentration of ions) and exist mainly in the form of molecules. Web.
You'll get a detailed solution from. Web 4 students found this answer helpful. Web which of the following is formed when (nh4)2s04 dissolves in water? This problem has been solved! Weak electrolytes, such as hgcl 2, conduct badly because they produce few ions when dissolved (low concentration of ions) and exist mainly in the form of molecules. Web about this tutor ›. Express your answers as ions separated by a. Web b) the compound ammonium sulfate, (nh 4) 2 so 4 is soluble in water. Enter the ions formed when (nh4)2s dissolves in water. Web chemistry for the compound \mathrm {li}_2 \mathrm {o} li2o, show that the charges on the ions add up to zero. Web science chemistry chemistry questions and answers enter the ions formed when (nh4)2 s dissolves in water. Web now, we take a closer look at reactions that include ionic compounds. The larger the concentration of ions, the better the solutions conducts. Web when the ionic compound #nh_4cl# dissolves in water, it breaks into one ammonium, #nh_4^+# and one. The ions formed are nh4 (+) and. Web solution for part b enter the ions formed when (nh4)2s dissolves in water. Thus, the nh 4+ will. Web an electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Write the net ionic equation for the. Web since hcl is a strong acid, ka is immeasurably large and kb ≈ 0 (chloride ions don’t undergo appreciable hydrolysis).
Write The Net Ionic Equation For The.
Web which of the following is formed when (nh4)2s04 dissolves in water? Web science chemistry chemistry questions and answers enter the ions formed when (nh4)2 s dissolves in water. Express your answers as ions separated by a. The larger the concentration of ions, the better the solutions conducts.
Nh 4 Br Is The Salt Of A Weak Base, Nh 4 Oh And A Strong Acid Hbr.
One important aspect about ionic compounds. Web which set of ions is formed when (nh4)2so4 dissolves in water? Web b) the compound ammonium sulfate, (nh 4) 2 so 4 is soluble in water. Thus, the nh 4+ will.
The Ions Formed Are Nh4 (+) And.
This problem has been solved! Web 4 students found this answer helpful. Enter the ions formed when (nh4)2s dissolves in water. Web when the ionic compound #nh_4cl# dissolves in water, it breaks into one ammonium, #nh_4^+# and one.
Weak Electrolytes, Such As Hgcl 2, Conduct Badly Because They Produce Few Ions When Dissolved (Low Concentration Of Ions) And Exist Mainly In The Form Of Molecules.
Web chemistry chemistry questions and answers enter the ions formed when (nh4)2 s dissolves in water. Web the salt ammonium sulfate dissolves in water according to the reaction: Web solution for part b enter the ions formed when (nh4)2s dissolves in water. Web an electrolyte solution conducts electricity because of the movement of ions in the solution (see above).