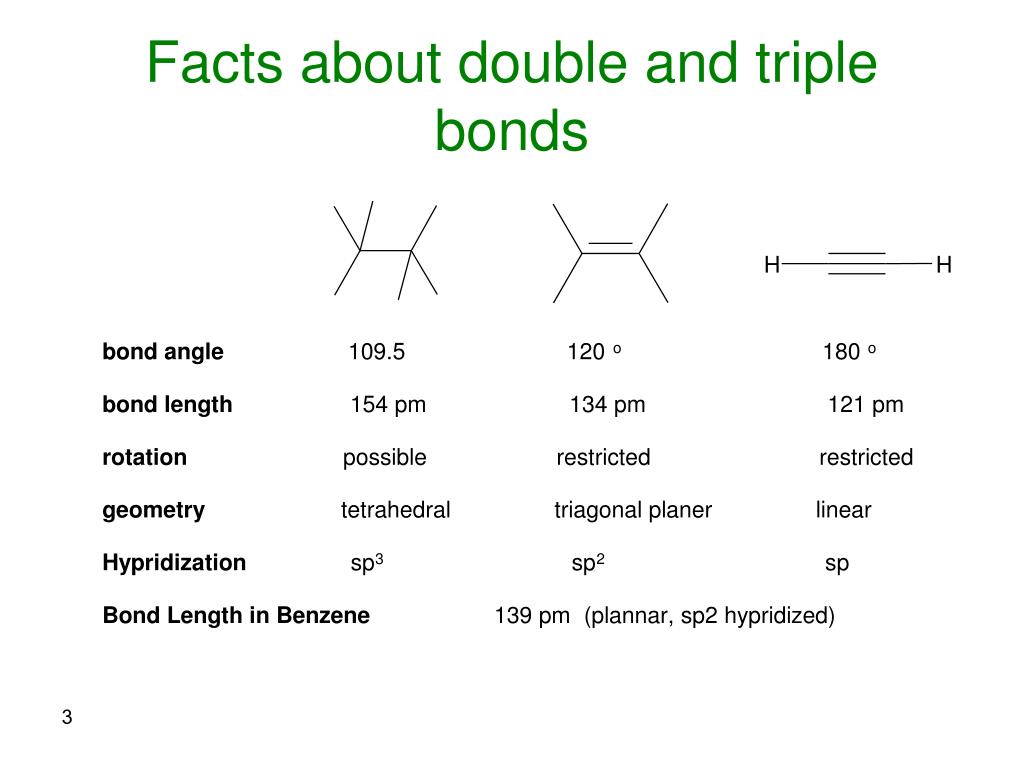
Double And Triple Bonds Form Because - Web double and triple bonds form because carbon which of the following elements does not exist as a diatomic molecule. Web science chemistry 6) double and triple bonds form because a. Web a double bond consists of two bonding electron pairs, four electrons shared between two atoms, so the bond order is 2. Covalent bond:.double lines (=) indicate a double bond between two atoms. Web a double bond is formed by the two atoms sharing two pairs of electrons. Double bonds form when the atoms share two pairs of electrons, and triple bonds form when they share three pairs. All bonds form as interactions of valence electrons of elements. The equals sign is used typographically for this. Web other articles where double bond is discussed: The atoms involved have high electronegativities.
PPT Organic chemistry for medicine and biology students Chem 2311
Web double and triple bonds form because carbon which of the following elements does not exist as a diatomic molecule. Double bonds form when the atoms share two pairs of electrons, and triple bonds form when they share three pairs. Web find the answer to question 8 about double and triple bonds formation from a subject matter expert on chegg.com..
PPT Valence Bond Theory PowerPoint Presentation ID5616566
Web other articles where double bond is discussed: Web a double bond consists of two bonding electron pairs, four electrons shared between two atoms, so the bond order is 2. This type of bond is stronger than a single. Web this video shows chemical bonds inside human body respiration & breathing. 5.0 (2 reviews) ) double and triple bonds form.
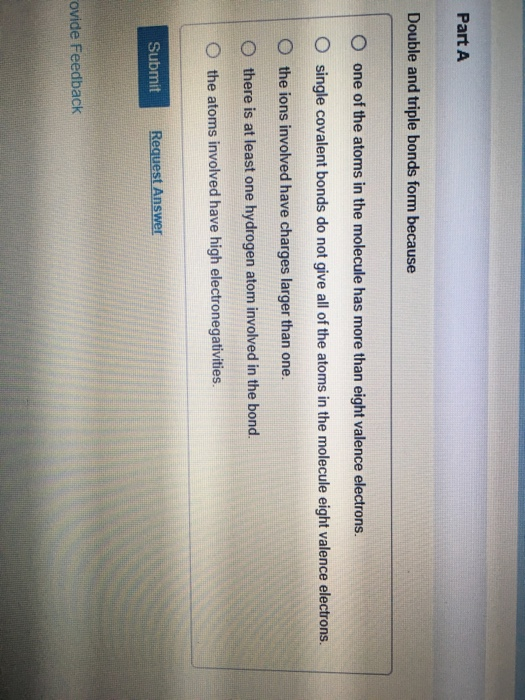
Solved Part A Double and triple bonds form because O one of
Double and triple bonds form because: Web answered • expert verified. Web a double bond forms when two pairs of electrons are shared between a pair of atoms, as between the carbon and oxygen. Double bonds form when the atoms share two pairs of electrons, and triple bonds form when they share three pairs. Web a double bond consists of.
PPT Valence Bond Theory PowerPoint Presentation ID5616566
Web find the answer to question 8 about double and triple bonds formation from a subject matter expert on chegg.com. Web this video shows chemical bonds inside human body respiration & breathing. Double bonds form when the atoms share two pairs of electrons, and triple bonds form when they share three pairs. Web a double bond is produced if two.
Multiple Bonds — Double & Triple Bonds Expii
Alexander butlerov, a russian chemist, was the first to use double bonds in chemical notation. Click the card to flip 👆. All of them are single pairing of. Web double and triple bonds can be explained by orbital hybridization, or the 'mixing' of atomic orbitals to form new hybrid orbitals. 5.0 (2 reviews) ) double and triple bonds form because.
PPT Notes 53 Covalent Bonds PowerPoint Presentation, free download
One of the atoms in the molecule has more. Web a double bond consists of two bonding electron pairs, four electrons shared between two atoms, so the bond order is 2. Double and triple bonds form because: All of them are single pairing of. Double bonds form when the atoms share two pairs of electrons, and triple bonds form when.
PPT Structure Determines Properties PowerPoint Presentation, free
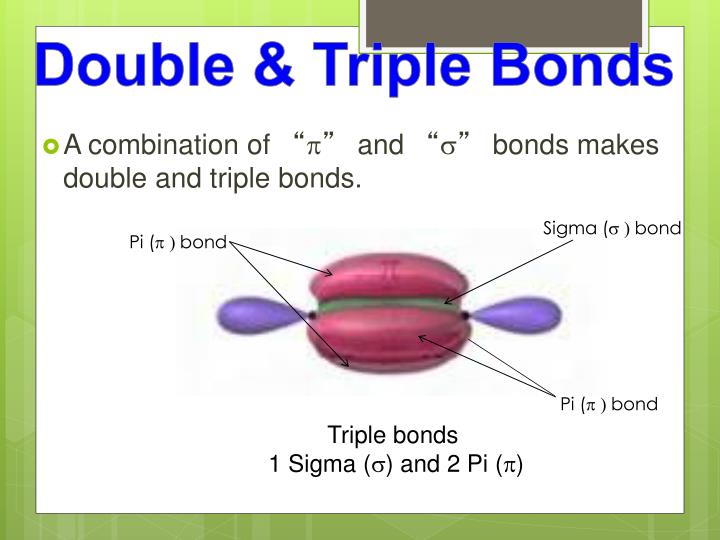
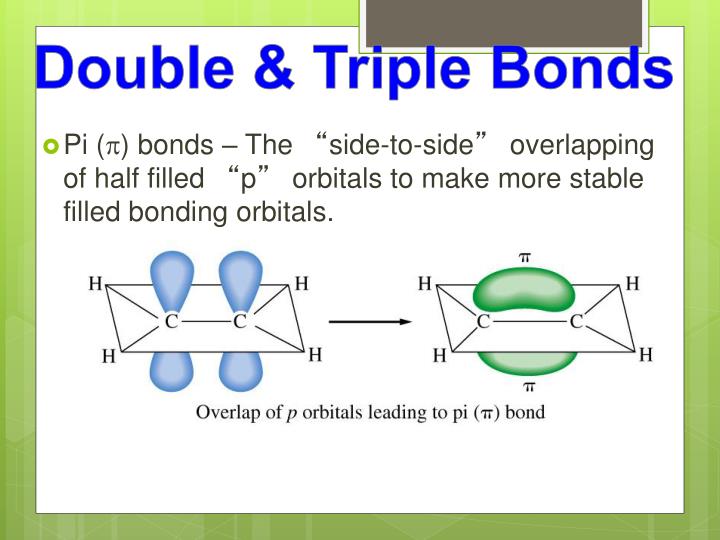
Web in \(\pi\) orbitals, the electron density lies above and below the axis connecting the bonded atoms. Covalent bond:.double lines (=) indicate a double bond between two atoms. Double and triple bonds form because: Part a double and triple bonds form because o one of the atoms in the molecule has more than eight valence electrons o. Web find the.
PPT Valence Bond Theory PowerPoint Presentation, free download ID
Web a double bond is produced if two shared pairs of electrons exist between the same pair of atoms, and a triple bond is created by. Web a double bond forms when two pairs of electrons are shared between a pair of atoms, as between the carbon and oxygen. The equals sign is used typographically for this. Web in \(\pi\).
PPT Organic Molecules The Building Blocks of Life PowerPoint
Web the answer is b) single covalent bonds do not give all of the atoms in the molecule eight valence electrons. Alexander butlerov, a russian chemist, was the first to use double bonds in chemical notation. This type of bond is stronger than a single. One of the atoms in the molecule has more. Double and triple bonds form because:
Organic compounds with both double and triple bonds YouTube
Web a double bond forms when two pairs of electrons are shared between a pair of atoms, as between the carbon and oxygen. Web double and triple bonds form because carbon which of the following elements does not exist as a diatomic molecule. The atoms involved have high electronegativities. Double and triple bonds form because: Alexander butlerov, a russian chemist,.
Single covalent bonds do not give all. Web find the answer to question 8 about double and triple bonds formation from a subject matter expert on chegg.com. Web other articles where double bond is discussed: All of them are single pairing of. 5.0 (2 reviews) ) double and triple bonds form because. Web double and triple bonds form because carbon which of the following elements does not exist as a diatomic molecule. All bonds form as interactions of valence electrons of elements. Double and triple bonds form because: Web the answer is b) single covalent bonds do not give all of the atoms in the molecule eight valence electrons. Web a double bond is depicted as two parallel lines (=) between the two connected atoms in a skeleton formula; Oxygen atoms can form double bonds, and. The atoms involved have high electronegativities. Alexander butlerov, a russian chemist, was the first to use double bonds in chemical notation. Web a double bond is formed by the two atoms sharing two pairs of electrons. The equals sign is used typographically for this. Covalent bond:.double lines (=) indicate a double bond between two atoms. Web single, double, and triple bonds are three types of covalent bonds mainly involving nonmetals. Click the card to flip 👆. Double bonds form when the atoms share two pairs of electrons, and triple bonds form when they share three pairs. Web double and triple bonds can be explained by orbital hybridization, or the 'mixing' of atomic orbitals to form new hybrid orbitals.
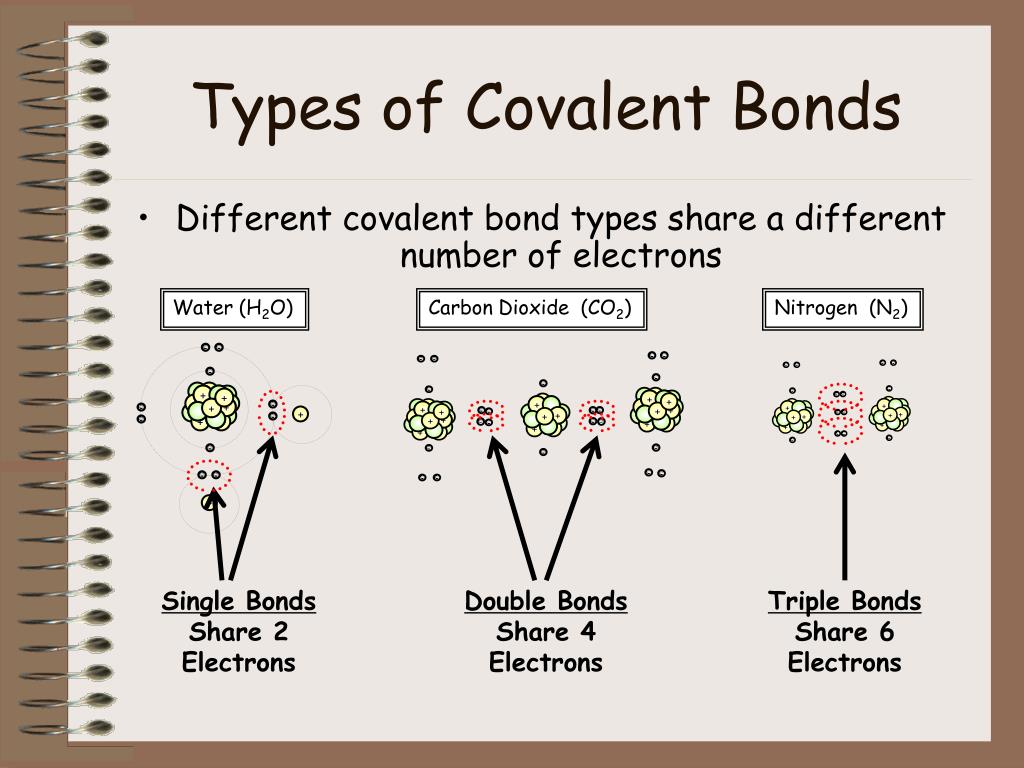
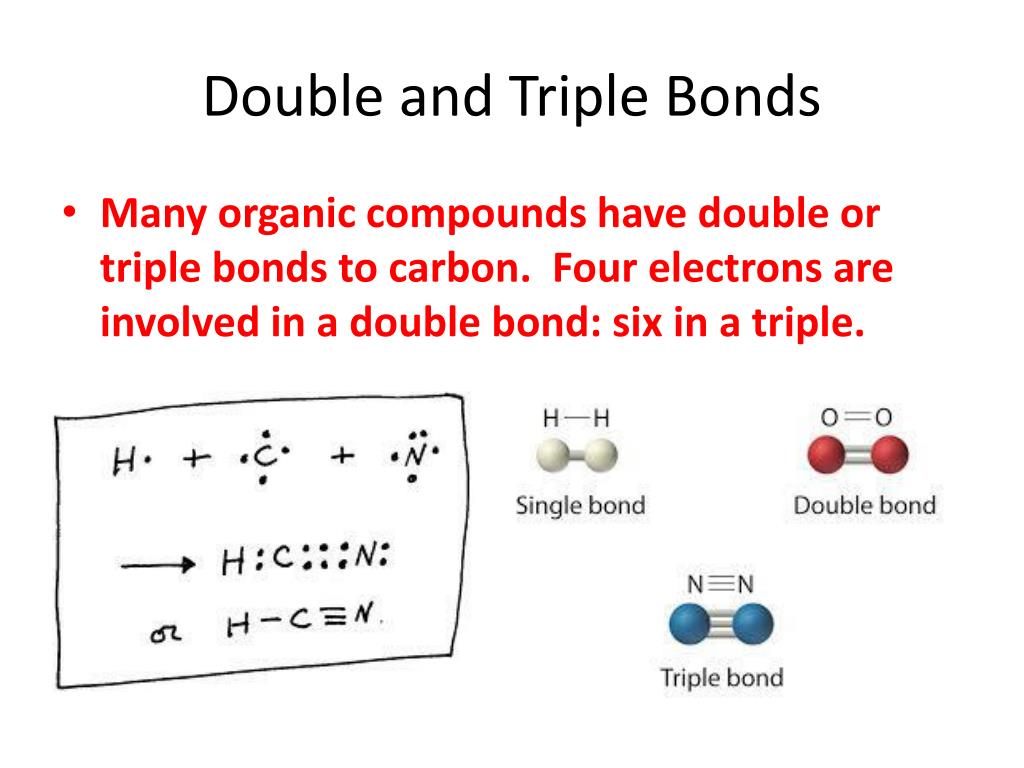
Double Bonds Form When The Atoms Share Two Pairs Of Electrons, And Triple Bonds Form When They Share Three Pairs.
Oxygen atoms can form double bonds, and. Web the answer is b) single covalent bonds do not give all of the atoms in the molecule eight valence electrons. Web find the answer to question 8 about double and triple bonds formation from a subject matter expert on chegg.com. This type of bond is stronger than a single.
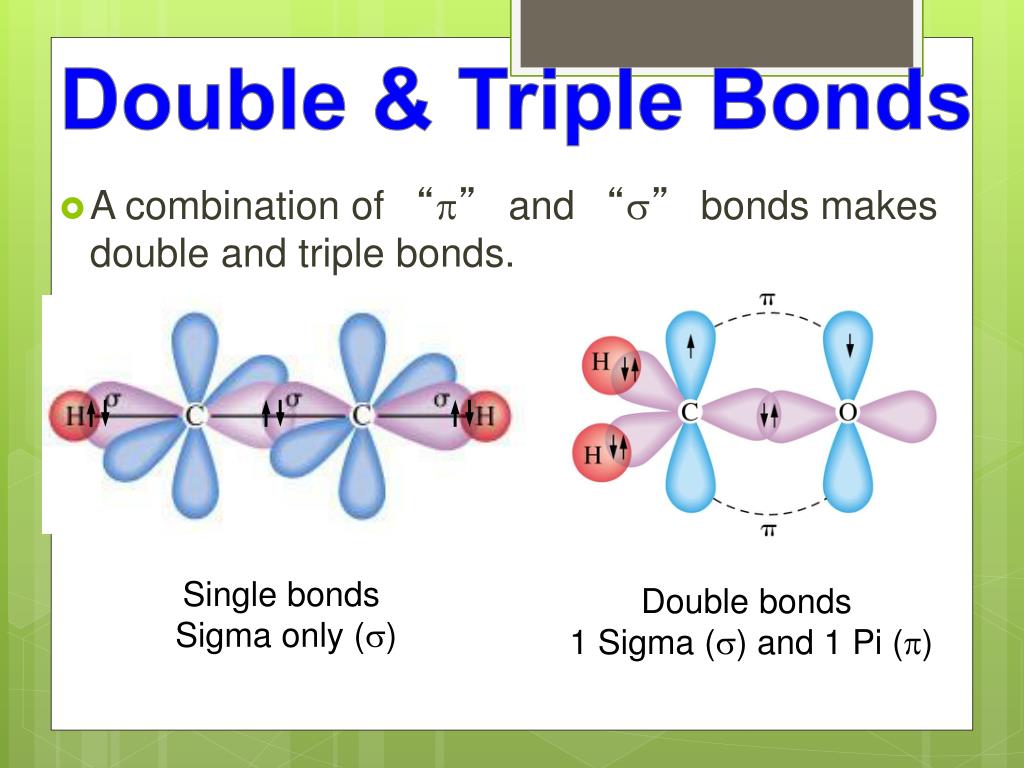
Web In \(\Pi\) Orbitals, The Electron Density Lies Above And Below The Axis Connecting The Bonded Atoms.
Web other articles where double bond is discussed: Web a double bond is formed by the two atoms sharing two pairs of electrons. Web science chemistry 6) double and triple bonds form because a. Web this video shows chemical bonds inside human body respiration & breathing.
The Atoms Involved Have High Electronegativities.
Alexander butlerov, a russian chemist, was the first to use double bonds in chemical notation. Web a double bond is depicted as two parallel lines (=) between the two connected atoms in a skeleton formula; Web double and triple bonds form because carbon which of the following elements does not exist as a diatomic molecule. Part a double and triple bonds form because o one of the atoms in the molecule has more than eight valence electrons o.
Web Double And Triple Bonds Can Be Explained By Orbital Hybridization, Or The 'Mixing' Of Atomic Orbitals To Form New Hybrid Orbitals.
All of them are single pairing of. Click the card to flip 👆. Web single, double, and triple bonds are three types of covalent bonds mainly involving nonmetals. Covalent bond:.double lines (=) indicate a double bond between two atoms.