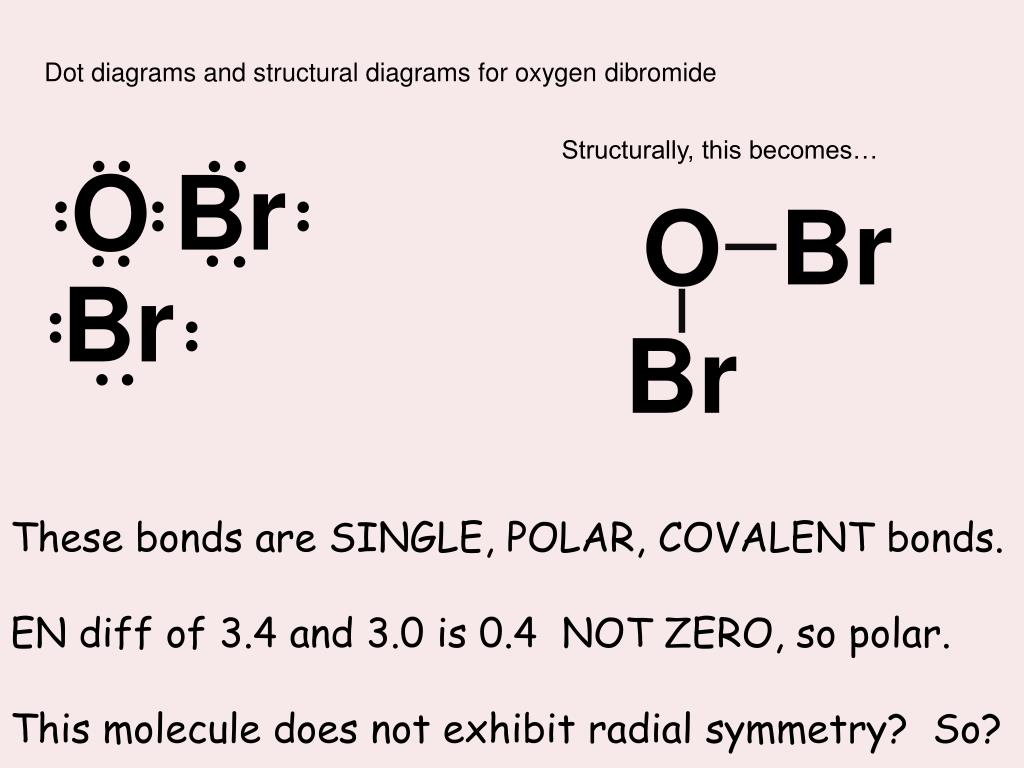
Does Oxygen And Bromine Form An Ionic Compound - The periodic table can help us. Bromine can react with elements from:. 3 can oxygen bond to. Web when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Example of ion charges and. Write a formula for the ionic compound that forms between each pair of elements. Web contents hide 1 is oxygen and bromine ionic or covalent? Web bromine compounds are compounds containing the element bromine (br). 2 does barium and oxygen form an ionic compound? We know that strontium is a group 2 element.
Formation of Ion SPM Chemistry
Example of ion charges and. It forms unstable yellow [2]. The periodic table can help us. From the answers we derive, we place the compound in an appropriate. Ions (chloride, bromide or iodide ions) are present.
PPT Ionic Bonding and Nomenclature PowerPoint Presentation, free
Web answer verified 221.4k + views hint: 1 the nature of the ionic bond. Web bromine dioxide is the chemical compound composed of bromine and oxygen with the formula bro 2. Nonmetals tend to form covalent. 2 does barium and oxygen form an ionic compound?
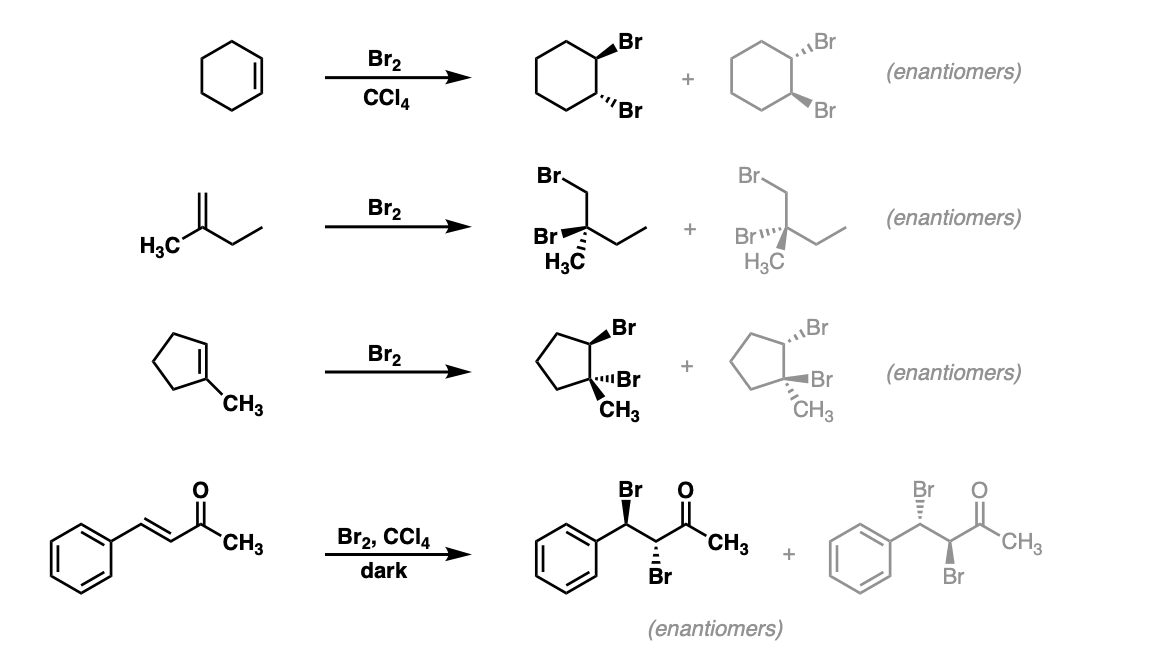
Bromination of alkenes with Br2 to give dibromides Master Organic
Web when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the. 1 the nature of the ionic bond. From the answers we derive, we place the compound in an appropriate. Web most metal bromides with the metal in low oxidation states (+1 to +3) are ionic. Nonmetals tend to form covalent.
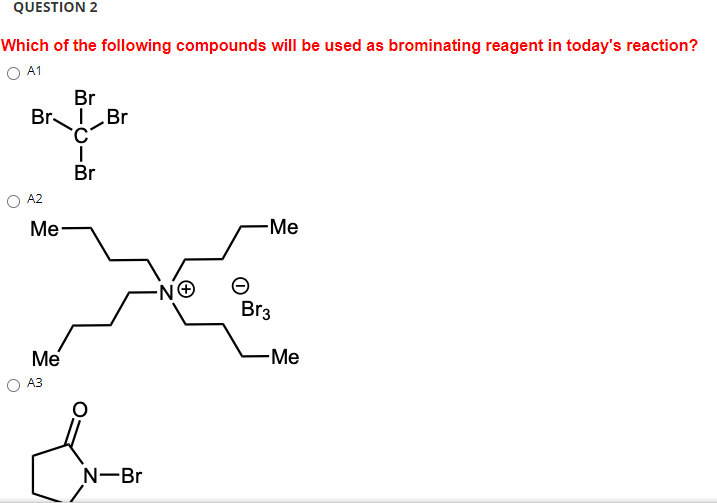
Solved QUESTION 2 Which of the following compounds will be
Write a formula for the ionic compound that forms between each pair of elements. Web when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the. 2 the crystal lattice structure of ionic. 1 the nature of the ionic bond. In the formula of an ionic compound we are showing the ratio.
Símbolos y estructuras de Lewis CHEM 1305 Introductory Chemistry El
Ions (chloride, bromide or iodide ions) are present. 2 the crystal lattice structure of ionic. In the formula of an ionic compound we are showing the ratio. From the answers we derive, we place the compound in an appropriate. Example of ion charges and.
Ionic Bonding Presentation Chemistry
Web when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Nonmetals tend to form covalent. Web if so, does it also contain oxygen? 2 the crystal lattice structure of ionic. Write a formula for the ionic compound that forms between each pair of elements.
organic chemistry Bromonium ion or Mesomeric effect ( intermediate
Web an ionic compound is made up of charged particles, called ions. An oxygen atom gains two electrons to form an oxide ion. Web oxygen is produced (from hydroxide ions), unless halide. Web bromine dioxide is the chemical compound composed of bromine and oxygen with the formula bro 2. 2 the crystal lattice structure of ionic.
Dot Diagram Ionic Bonds Images Amashusho
Web therefore, bromine needs to gain one electron in order to become stable. 2 does barium and oxygen form an ionic compound? Web an oxygen atom gains two electrons to form an oxide ion ions are formed by the transfer of electrons. It forms unstable yellow [2]. Web therefore, it is most likely an ionic compound (in fact, it is.
PPT Bonding Class 4 PowerPoint Presentation, free download ID3953863
It forms unstable yellow [2]. From the answers we derive, we place the compound in an appropriate. Nonmetals tend to form covalent. 3 can oxygen bond to. Web when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge.
The top panel in this figure shows two hydrogen atoms sharing two
Web when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the. Nonmetals tend to form covalent. 1 the nature of the ionic bond. Web therefore, it is most likely an ionic compound (in fact, it is ionic). In contrast, the compound \(\ce{no2}\) contains two.
Web bromine dioxide is the chemical compound composed of bromine and oxygen with the formula bro 2. Web when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the. 2 the crystal lattice structure of ionic. Web contents hide 1 is oxygen and bromine ionic or covalent? Web answer verified 221.4k + views hint: 1 the nature of the ionic bond. Write a formula for the ionic compound that forms between each pair of elements. In the formula of an ionic compound we are showing the ratio. Web an ionic compound is made up of charged particles, called ions. Example of ion charges and. In contrast, the compound \(\ce{no2}\) contains two. Web oxygen is produced (from hydroxide ions), unless halide. Web physical sciences grade 10. From the answers we derive, we place the compound in an appropriate. It forms unstable yellow [2]. The periodic table can help us. 2 does barium and oxygen form an ionic compound? An oxygen atom gains two electrons to form an oxide ion. Web when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Web if so, does it also contain oxygen?
Web Most Metal Bromides With The Metal In Low Oxidation States (+1 To +3) Are Ionic.
Ions (chloride, bromide or iodide ions) are present. Bromine can react with elements from:. We know that strontium is a group 2 element. 2 the crystal lattice structure of ionic.
Web A Compound That Contains Ions And Is Held Together By Ionic Bonds Is Called An Ionic Compound.
Web contents hide 1 is oxygen and bromine ionic or covalent? Web therefore, it is most likely an ionic compound (in fact, it is ionic). Web therefore, bromine needs to gain one electron in order to become stable. 2 does barium and oxygen form an ionic compound?
In The Formula Of An Ionic Compound We Are Showing The Ratio.
An oxygen atom gains two electrons to form an oxide ion. Web bromine compounds are compounds containing the element bromine (br). It forms unstable yellow [2]. Web the difference in electronegativity between oxygen and hydrogen is not small.
Web Bromine Dioxide Is The Chemical Compound Composed Of Bromine And Oxygen With The Formula Bro 2.
Write a formula for the ionic compound that forms between each pair of elements. Web an ionic compound is made up of charged particles, called ions. Web an oxygen atom gains two electrons to form an oxide ion ions are formed by the transfer of electrons. Web when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the.




.PNG)