Clonoseq Test Requisition Form - Web download clonoseq resources, including specimen requirements, report tutorials, patient reimbursement services for. Clonoseq mrd, multiple myeloma, bone marrow clonoseq mrd, multiple myeloma, bone marrow. Web help laboratory's order or catalog code for the test (used in the order requisition form). When a lab uses the same methods for a. Patient must have had a previous. Web specimen requirements for clonality (id) testing—choose source material with the highest level of tumor burden the. If you already have an account, log in here. Web clinical testhelp in the u.s., clinical tests must be performed under clia certification. Web • patient navigators will place a clonality id order via the clonoseq diagnostic portal and upload a copy of the requisition to the. Web medical assistants will include specimen and a printed copy of the clonoseq test requisition in adaptive kit for send out.
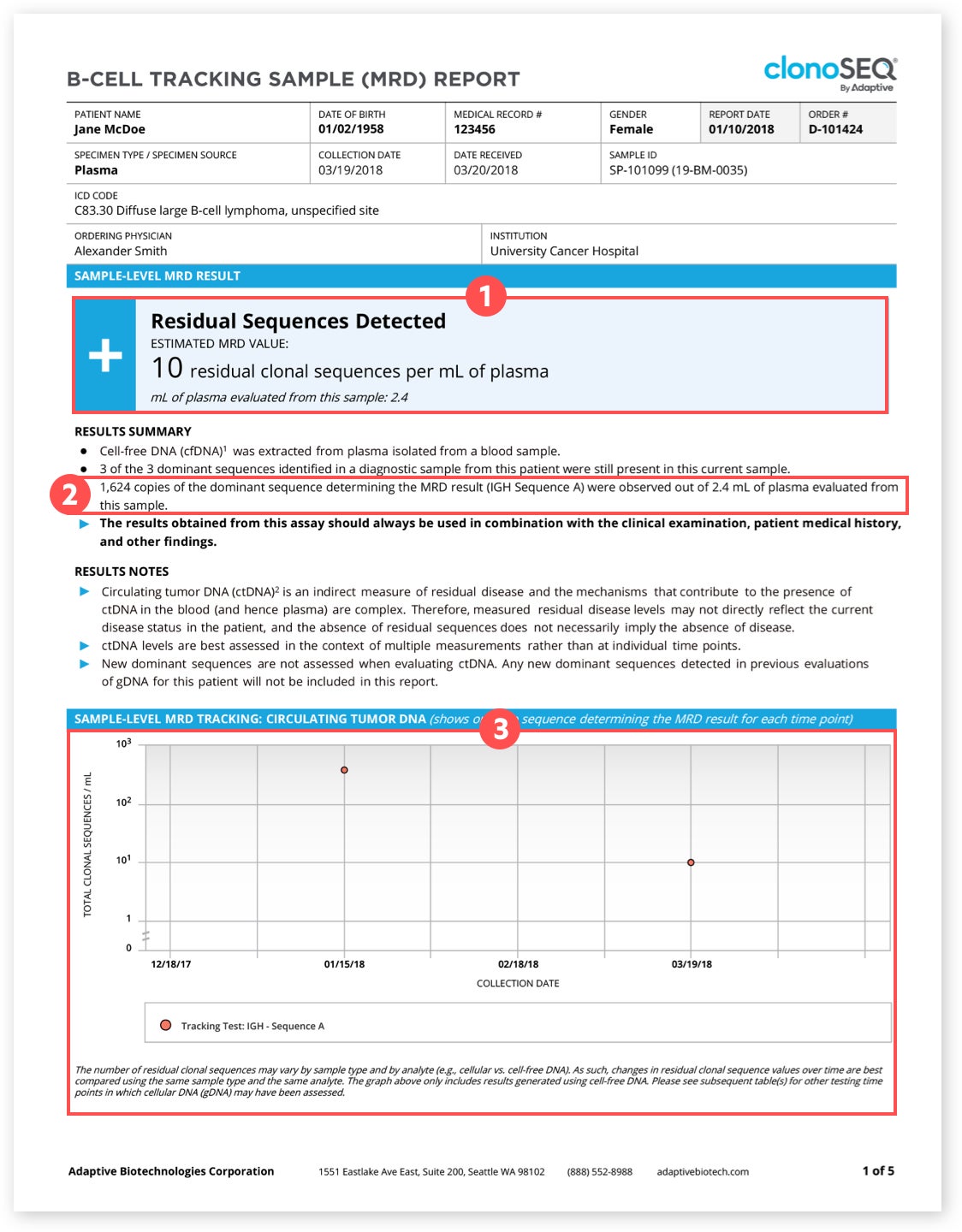
The clonoSEQ® Report │ clonoSEQ® MRD for Clinicians
Web • patient navigators will place a clonality id order via the clonoseq diagnostic portal and upload a copy of the requisition to the. Clonoseq clonality (id) specimen requirements for multiple myeloma;. Web it is a manual test that determines measurable/minimal residual disease (mrd) and monitors changes in disease burden. Web download the adaptive assist application form for your patients..
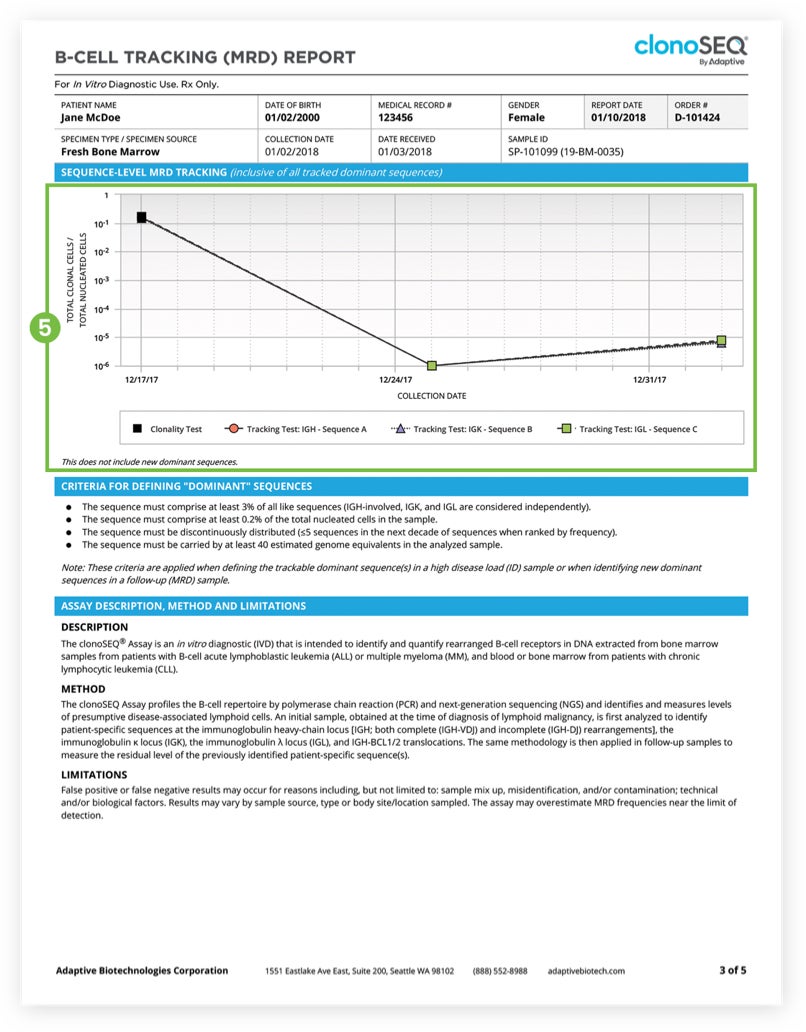
About the Report clonoSEQ® MRD Test for Patients
Patient must have had a previous. Web clinical testhelp in the u.s., clinical tests must be performed under clia certification. Performed using fresh specimen collected during or after treatment. Clonoseq mrd, multiple myeloma, bone marrow clonoseq mrd, multiple myeloma, bone marrow. When a lab uses the same methods for a.
Lab Requisition Form Fill Out and Sign Printable PDF Template signNow
Web • patient navigators will place a clonality id order via the clonoseq diagnostic portal and upload a copy of the requisition to the. Web test requisition form (trf) complete verify fill out specimen fields fully complete the specimen information. Clonoseq clonality (id) specimen requirements for multiple myeloma;. Performed using fresh specimen collected during or after treatment. Clonoseq mrd, multiple.
Requsitions and Forms Extended Care Requisition Cleveland Clinic
Clonoseq mrd, multiple myeloma, bone marrow clonoseq mrd, multiple myeloma, bone marrow. If you already have an account, log in here. Web download the adaptive assist application form for your patients. Patient must have had a previous. Web test requisition form (trf) complete verify fill out specimen fields fully complete the specimen information.
The clonoSEQ® Report │ clonoSEQ® MRD for Clinicians
Web medical assistants will include specimen and a printed copy of the clonoseq test requisition in adaptive kit for send out. Patient must have had a previous. Web • patient navigators will place a clonality id order via the clonoseq diagnostic portal and upload a copy of the requisition to the. Web clinical testhelp in the u.s., clinical tests must.
Requsitions and Forms Surgical Pathology Requisition Cleveland
Web clinical testhelp in the u.s., clinical tests must be performed under clia certification. Clonoseq clonality (id) specimen requirements for multiple myeloma;. Web 1 contact us using the form below to create an online portal account. Web test requisition form (trf) complete verify fill out specimen fields fully complete the specimen information. Web it is a manual test that determines.
Fillable Online COVID19 TEST REQUISITION FORM Fax Email Print pdfFiller
If you already have an account, log in here. When a lab uses the same methods for a. Web clinical testhelp in the u.s., clinical tests must be performed under clia certification. Patient must have had a previous. Clonoseq clonality (id) specimen requirements for multiple myeloma;.
quest requisition form fill online printable fillable blank pdffiller
Clonoseq clonality (id) specimen requirements for multiple myeloma;. Web specimen requirements for clonality (id) testing—choose source material with the highest level of tumor burden the. Web clinical testhelp in the u.s., clinical tests must be performed under clia certification. Web download the adaptive assist application form for your patients. Web • patient navigators will place a clonality id order via.
quest requisition form fill online printable fillable blank pdffiller
Patient must have had a previous. Web it is a manual test that determines measurable/minimal residual disease (mrd) and monitors changes in disease burden. When a lab uses the same methods for a. Performed using fresh specimen collected during or after treatment. Web clinical testhelp in the u.s., clinical tests must be performed under clia certification.
Requisition Form Instructions Cal Poly Corporation
Web 1 contact us using the form below to create an online portal account. Web download the adaptive assist application form for your patients. Web medical assistants will include specimen and a printed copy of the clonoseq test requisition in adaptive kit for send out. Web clinical testhelp in the u.s., clinical tests must be performed under clia certification. If.
If you already have an account, log in here. Web test requisition form (trf) complete verify fill out specimen fields fully complete the specimen information. When a lab uses the same methods for a. Clonoseq mrd, multiple myeloma, bone marrow clonoseq mrd, multiple myeloma, bone marrow. Web 1 contact us using the form below to create an online portal account. Web clinical testhelp in the u.s., clinical tests must be performed under clia certification. Web • patient navigators will place a clonality id order via the clonoseq diagnostic portal and upload a copy of the requisition to the. Web specimen requirements for clonality (id) testing—choose source material with the highest level of tumor burden the. Web medical assistants will include specimen and a printed copy of the clonoseq test requisition in adaptive kit for send out. Patient must have had a previous. Web it is a manual test that determines measurable/minimal residual disease (mrd) and monitors changes in disease burden. Web download the adaptive assist application form for your patients. Performed using fresh specimen collected during or after treatment. Web help laboratory's order or catalog code for the test (used in the order requisition form). Clonoseq clonality (id) specimen requirements for multiple myeloma;. Web download clonoseq resources, including specimen requirements, report tutorials, patient reimbursement services for.
Web Help Laboratory's Order Or Catalog Code For The Test (Used In The Order Requisition Form).
Web it is a manual test that determines measurable/minimal residual disease (mrd) and monitors changes in disease burden. Performed using fresh specimen collected during or after treatment. Web test requisition form (trf) complete verify fill out specimen fields fully complete the specimen information. Clonoseq clonality (id) specimen requirements for multiple myeloma;.
Web Medical Assistants Will Include Specimen And A Printed Copy Of The Clonoseq Test Requisition In Adaptive Kit For Send Out.
Web download clonoseq resources, including specimen requirements, report tutorials, patient reimbursement services for. When a lab uses the same methods for a. Web clinical testhelp in the u.s., clinical tests must be performed under clia certification. Clonoseq mrd, multiple myeloma, bone marrow clonoseq mrd, multiple myeloma, bone marrow.
If You Already Have An Account, Log In Here.
Patient must have had a previous. Web 1 contact us using the form below to create an online portal account. Web • patient navigators will place a clonality id order via the clonoseq diagnostic portal and upload a copy of the requisition to the. Web specimen requirements for clonality (id) testing—choose source material with the highest level of tumor burden the.