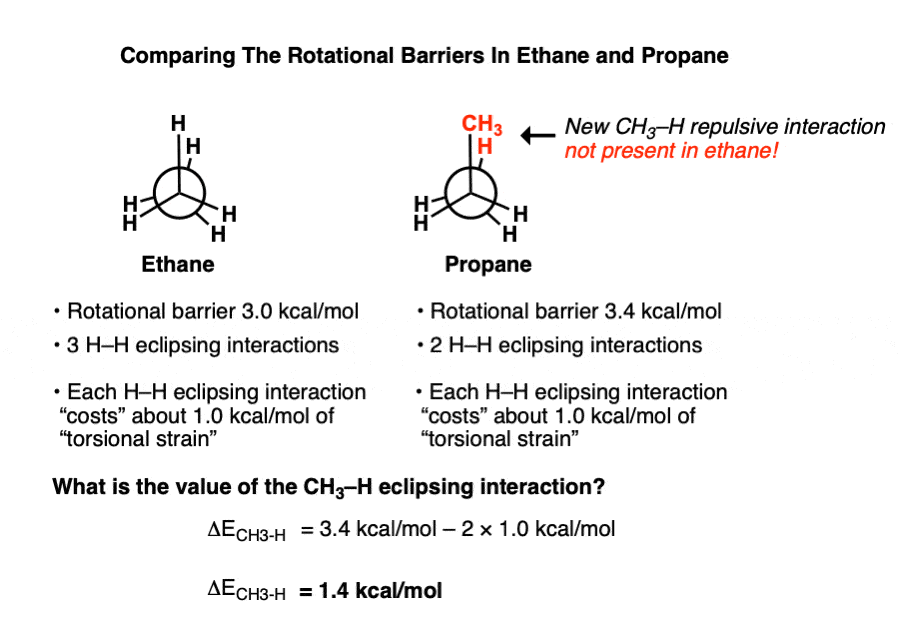
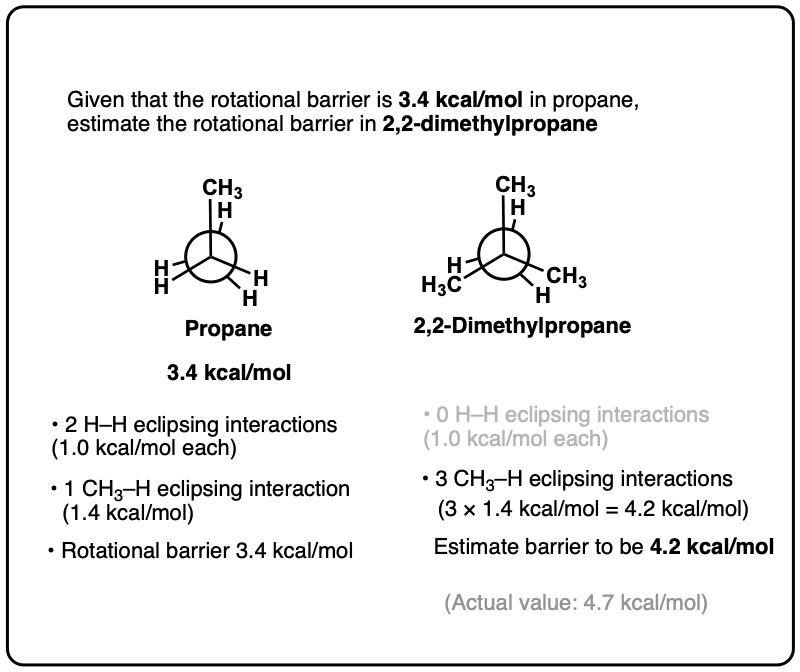
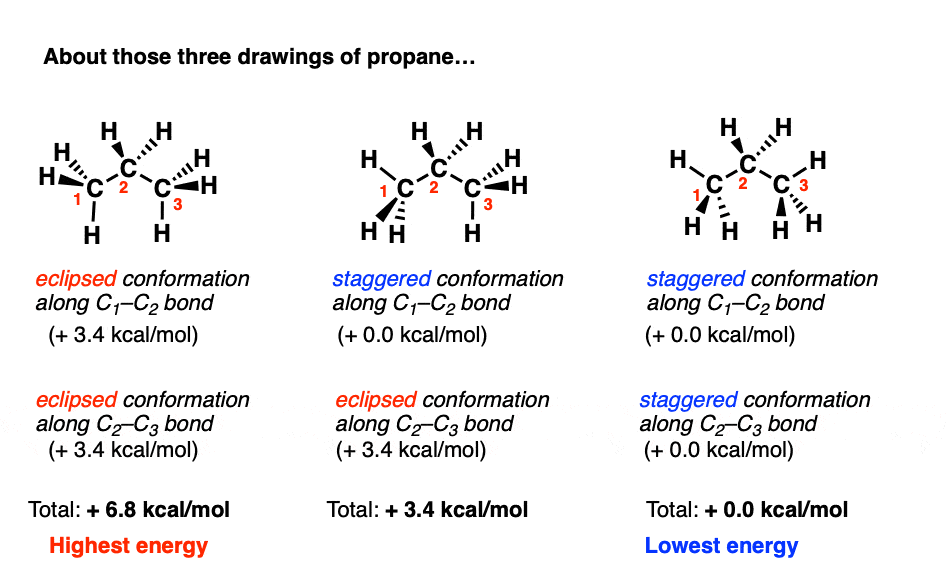
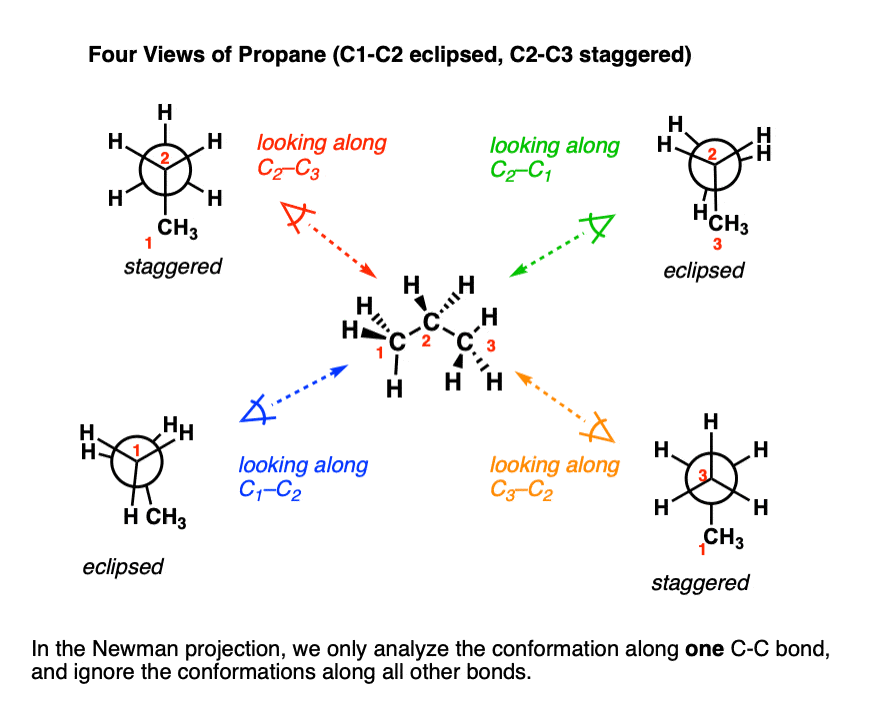
Can Propane Form Isomers - Web in chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted just by. December 13th, 2022 | the conformational isomers of propane (are awesome) in this post we’ll explore the different conformations of propane and see that it has a slightly higher barrier to rotation than ethane due to a new steric interaction. A) name and show the full. Both have a chain of three carbon. Web yes we can, above are the isomers of propane. Web when propane reacts with chlorine, two different monochloropropanes are formed. Web conformational isomers of propane last updated: Web solution isomers are defined as those species which possess similar chemical formulas but different structural formulas. Web solution for visual skills see figures 4.5a and 4.7. Why do alkanes with more than two carbons have a kinked structure?.
Conformational Isomers of Propane Master Organic Chemistry
Web first, let's draw the structure of propane and see if we can draw some isomers: Web solution isomers are defined as those species which possess similar chemical formulas but different structural formulas. Methane (ch 4 ), ethane (c 2 h 6 ), and. Web conformational isomers of propane last updated: Web click here👆to get an answer to your question.
Propane Propane Structural Formula
Web yes we can, above are the isomers of propane. Web in chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted just by. Web propane does not have any functional groups and hence can't have any position isomers. Web propane cannot form structural isomers because the three carbons atoms can attach only in a.
Conformational Isomers of Propane Master Organic Chemistry
Unlike conformational isomers, which do not differ in connectivity, structural isomers differ in connectivity,. December 13th, 2022 | the conformational isomers of propane (are awesome) in this post we’ll explore the different conformations of propane and see that it has a slightly higher barrier to rotation than ethane due to a new steric interaction. Web propane cannot form structural isomers.
Conformers of Propane Conformational isomerism Staggered
Web propane cannot form structural isomers because the three carbons atoms can attach only in a straight line. Web the first two isomers shown of are propanols, that is, alcohols derived from propane. Web first, let's draw the structure of propane and see if we can draw some isomers: Web propane’s structure demonstrates that it does not contain enough carbon.
Conformational Isomers of Propane LaptrinhX / News
Web even if two hydrocarbons have the same molecular formula, their atoms may be connected or oriented in different ways, making them isomers of one another. Web yes we can, above are the isomers of propane. Web the first two isomers shown of are propanols, that is, alcohols derived from propane. Propane is a molecule that contains three carbon atoms..
CH105 Chapter 7 Alkanes and Halogenated Hydrocarbons Chemistry
Does the compound have optical isomers? Both have a chain of three carbon. Web yes we can, above are the isomers of propane. Unlike conformational isomers, which do not differ in connectivity, structural isomers differ in connectivity,. Web when propane reacts with chlorine, two different monochloropropanes are formed.
draw the structure of ISO propane Brainly.in
Web propane (c₃h₈) is a saturated hydrocarbon that contains no double bonds between its carbons. If the compound has optical. Web even if two hydrocarbons have the same molecular formula, their atoms may be connected or oriented in different ways, making them isomers of one another. Web propane cannot form structural isomers because the three carbons atoms can attach only.
Conformational Isomers of Propane Master Organic Chemistry
Web solution for visual skills see figures 4.5a and 4.7. Why do alkanes with more than two carbons have a kinked structure?. Web solution isomers are defined as those species which possess similar chemical formulas but different structural formulas. Web conformational isomers of propane last updated: Both have a chain of three carbon.
Conformational Isomers of Propane Master Organic Chemistry
As we can see from the structure, we can't draw any. Web solution isomers are defined as those species which possess similar chemical formulas but different structural formulas. Propane is a molecule that contains three carbon atoms. Web even if two hydrocarbons have the same molecular formula, their atoms may be connected or oriented in different ways, making them isomers.
Conformational isomers of Propane and Butane YouTube
Web first, let's draw the structure of propane and see if we can draw some isomers: Web solution for visual skills see figures 4.5a and 4.7. Propane is a molecule that contains three carbon atoms. They must be on the outside of the carbon. Web propane cannot form structural isomers because the three carbons atoms can attach only in a.
A) name and show the full. Web propane cannot form structural isomers because the three carbons atoms can attach only in a straight line. December 13th, 2022 | the conformational isomers of propane (are awesome) in this post we’ll explore the different conformations of propane and see that it has a slightly higher barrier to rotation than ethane due to a new steric interaction. At the same time since. Web solution isomers are defined as those species which possess similar chemical formulas but different structural formulas. Web propane does not have any functional groups and hence can't have any position isomers. Web solution for visual skills see figures 4.5a and 4.7. Web in chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted just by. If the compound has optical. As we can see from the structure, we can't draw any. Web even if two hydrocarbons have the same molecular formula, their atoms may be connected or oriented in different ways, making them isomers of one another. Unlike conformational isomers, which do not differ in connectivity, structural isomers differ in connectivity,. Web the first two isomers shown of are propanols, that is, alcohols derived from propane. Why do alkanes with more than two carbons have a kinked structure?. Web when propane reacts with chlorine, two different monochloropropanes are formed. Web propane’s structure demonstrates that it does not contain enough carbon atoms to exist as a branching isomer. Propane is a molecule that contains three carbon atoms. Web does propane have any isomers? Does the compound have optical isomers? Web first, let's draw the structure of propane and see if we can draw some isomers:
Web Even If Two Hydrocarbons Have The Same Molecular Formula, Their Atoms May Be Connected Or Oriented In Different Ways, Making Them Isomers Of One Another.
Both have a chain of three carbon. Web because hydrogen can form one bond, the hydrogen can not be placed between two carbon atoms. If the compound has optical. Web first, let's draw the structure of propane and see if we can draw some isomers:
Propane Is A Molecule That Contains Three Carbon Atoms.
As we can see from the structure, we can't draw any. Web conformational isomers of propane last updated: Web solution isomers are defined as those species which possess similar chemical formulas but different structural formulas. Web the first two isomers shown of are propanols, that is, alcohols derived from propane.
Web Yes We Can, Above Are The Isomers Of Propane.
December 13th, 2022 | the conformational isomers of propane (are awesome) in this post we’ll explore the different conformations of propane and see that it has a slightly higher barrier to rotation than ethane due to a new steric interaction. Web does propane have any isomers? Web propane does not have any functional groups and hence can't have any position isomers. Web propane cannot form structural isomers because the three carbons atoms can attach only in a straight line.
Web In Chemistry, Conformational Isomerism Is A Form Of Stereoisomerism In Which The Isomers Can Be Interconverted Just By.
Web propane’s structure demonstrates that it does not contain enough carbon atoms to exist as a branching isomer. They must be on the outside of the carbon. Web click here👆to get an answer to your question ️ the number of isomers possible for propane is 2. A) name and show the full.