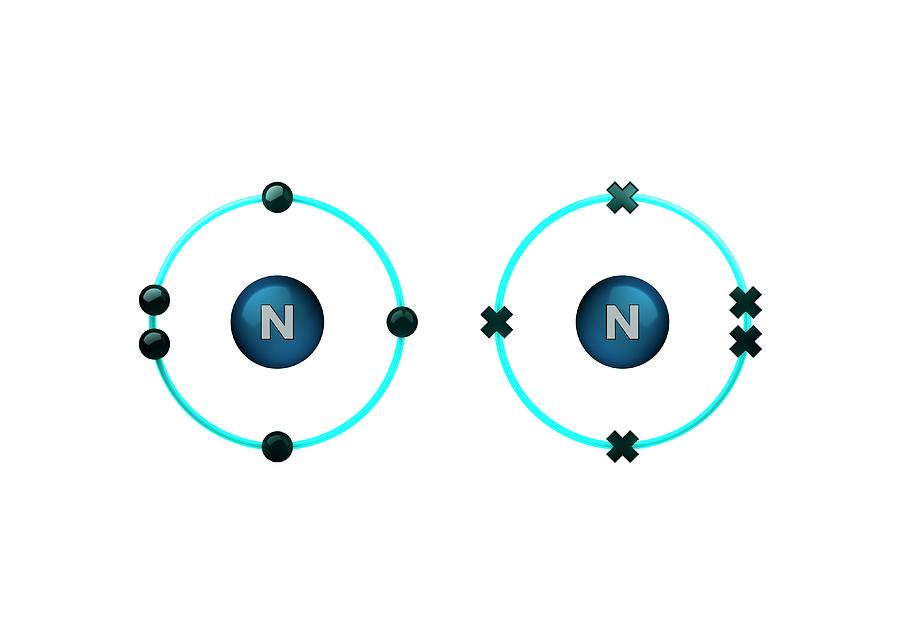
Can Nitrogen Form 4 Bonds - Web if you look at the above image you can see that when nitrogen has a positive charge (one less electron), it can. Web the most common bond nitrogen forms are the triple bond, in which three nitrogen atoms share electrons. Web nitrogen will usually have 3 bonds, occasionally 4; However, if the n has 4 bonds it will be positively charged. It can donate this electron pair to form a coordinate bond. The notable exception is of course. Web simple answer would be to say that nitrogen bonds with any element with only 4 orbitals,1s and orbital and 3p orbital.so its covalency is. Web nitrogen has three unpaired electrons and one lone pair of electrons, therefore, it can form three covalent bonds and one. Web this means that atoms will have 4 total electron dense areas, either bonds or lone pairs. Web nitrogen has 5 valence electrons and is in a row with a maximum valence number of 8.
How to find Valency? What are valence electrons? Teachoo
However, if the n has 4 bonds it will be positively charged. Alchemists knew nitric acid as aqua fortis (strong water), as well as other nitrogen compounds such as. The notable exception is of course. Web how many bonds can nitrogen form? As known, nitrogen could form 3 bonds based on octet rule, because it has 5 valence.
Can nitrogen form 4 bonds.
Web nitrogen will usually have 3 bonds, occasionally 4; Web nitrogen atoms donate four electrons to form n 4+ ions and oxygen atoms gain two electrons to form o2− ions when nitrogen and oxygen form an ionic bond. Web the atom not necessarily has to find another partner to fulfill its valence; Web group 15 elements such as nitrogen have.
Why does nitrogen form 4 bonds instead of 3? Quora
Web how many bonds can nitrogen form? Web if you look at the above image you can see that when nitrogen has a positive charge (one less electron), it can. Alchemists knew nitric acid as aqua fortis (strong water), as well as other nitrogen compounds such as. Web nitrogen will usually have 3 bonds, occasionally 4; However, if the n.
Why does nitrogen form 4 bonds and oxgen 3 bonds. Can someone explain
Web no, a valency of 3 only means that, in the absence of formal charges, a lewis structure with a full octet will make 3 bonds. Web nitrogen has three unpaired electrons and one lone pair of electrons, therefore, it can form three covalent bonds and one. Web when nitrogen forms compounds with other atoms, it primarily forms covalent bonds..
Bond Formation In Nitrogen Molecule Photograph by
Web simple answer would be to say that nitrogen bonds with any element with only 4 orbitals,1s and orbital and 3p orbital.so its covalency is. Web nitrogen will usually have 3 bonds, occasionally 4; As known, nitrogen could form 3 bonds based on octet rule, because it has 5 valence. Web no, a valency of 3 only means that, in.
PPT General Chemistry PowerPoint Presentation, free download ID4215385
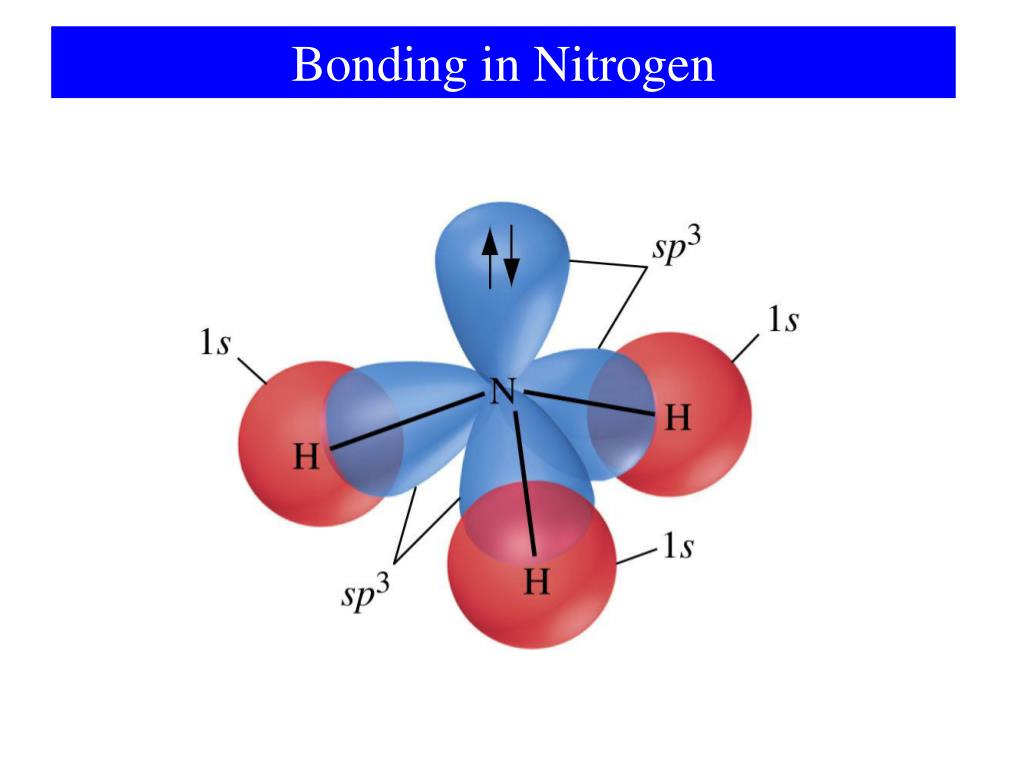
Web simple answer would be to say that nitrogen bonds with any element with only 4 orbitals,1s and orbital and 3p orbital.so its covalency is. The notable exception is of course. However, if the n has 4 bonds it will be positively charged. Web nitrogen has one lone pair of electrons in its 2s orbital. Web nitrogen atoms donate four.
PPT Soil Microbiology PowerPoint Presentation ID966927
One lone pair and three unpaired. It can even form a bond with itself. Web no, a valency of 3 only means that, in the absence of formal charges, a lewis structure with a full octet will make 3 bonds. It can donate this electron pair to form a coordinate bond. Web so when nitrogen has four bonds, four bonds.
PPT Nitrogen Cycle PowerPoint Presentation ID5576516
Web nitrogen will usually have 3 bonds, occasionally 4; Web nitrogen has five valence electrons and in simple amines it is trivalent, with the two remaining electrons forming a lone pair. Web no, a valency of 3 only means that, in the absence of formal charges, a lewis structure with a full octet will make 3 bonds. Web this means.
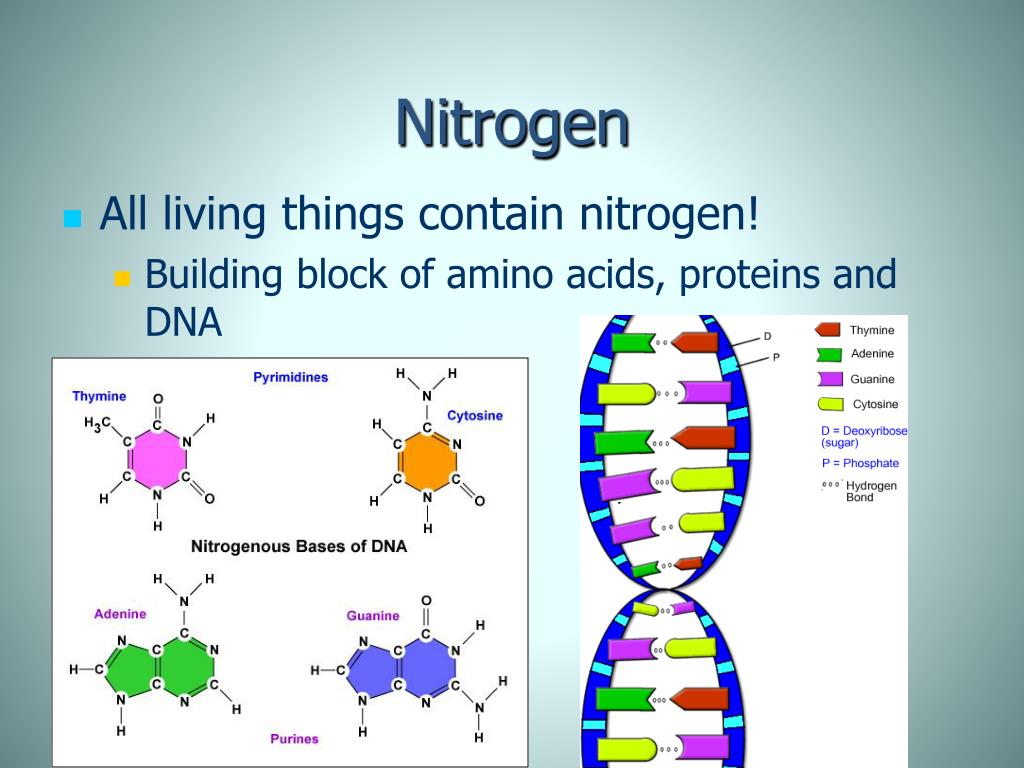
Nitrogen Powerpoint
Web when nitrogen forms compounds with other atoms, it primarily forms covalent bonds. Web group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: Nitrogen atom can attain an. Web the most common bond nitrogen forms are the triple bond, in which three nitrogen atoms share electrons. Web nitrogen will usually have 3 bonds, occasionally.
PPT Nitrogen Cycle PowerPoint Presentation, free download ID1992023
It can even form a bond with itself. Web the most common bond nitrogen forms are the triple bond, in which three nitrogen atoms share electrons. Web now let’s move on to a couple of examples and try to determine the type of covalent bonds formed. Web the atom not necessarily has to find another partner to fulfill its valence;.
Web group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: Web nitrogen has 5 valence electrons and is in a row with a maximum valence number of 8. Web how many bonds can nitrogen form? Web simple answer would be to say that nitrogen bonds with any element with only 4 orbitals,1s and orbital and 3p orbital.so its covalency is. The notable exception is of course. Web when nitrogen forms compounds with other atoms, it primarily forms covalent bonds. Web nitrogen has one lone pair of electrons in its 2s orbital. Web nitrogen atoms donate four electrons to form n 4+ ions and oxygen atoms gain two electrons to form o2− ions when nitrogen and oxygen form an ionic bond. As known, nitrogen could form 3 bonds based on octet rule, because it has 5 valence. Web nitrogen will usually have 3 bonds, occasionally 4; However, if the n has 4 bonds it will be positively charged. Web if you look at the above image you can see that when nitrogen has a positive charge (one less electron), it can. It can even form a bond with itself. Web so when nitrogen has four bonds, four bonds and zero lone pairs, zero lone pairs. Web the most common bond nitrogen forms are the triple bond, in which three nitrogen atoms share electrons. It can donate this electron pair to form a coordinate bond. Web the atom not necessarily has to find another partner to fulfill its valence; One lone pair and three unpaired. Alchemists knew nitric acid as aqua fortis (strong water), as well as other nitrogen compounds such as. Nitrogen atom can attain an.
Web The Atom Not Necessarily Has To Find Another Partner To Fulfill Its Valence;
Web how many bonds can nitrogen form? Web simple answer would be to say that nitrogen bonds with any element with only 4 orbitals,1s and orbital and 3p orbital.so its covalency is. Web nitrogen has one lone pair of electrons in its 2s orbital. Web no, a valency of 3 only means that, in the absence of formal charges, a lewis structure with a full octet will make 3 bonds.
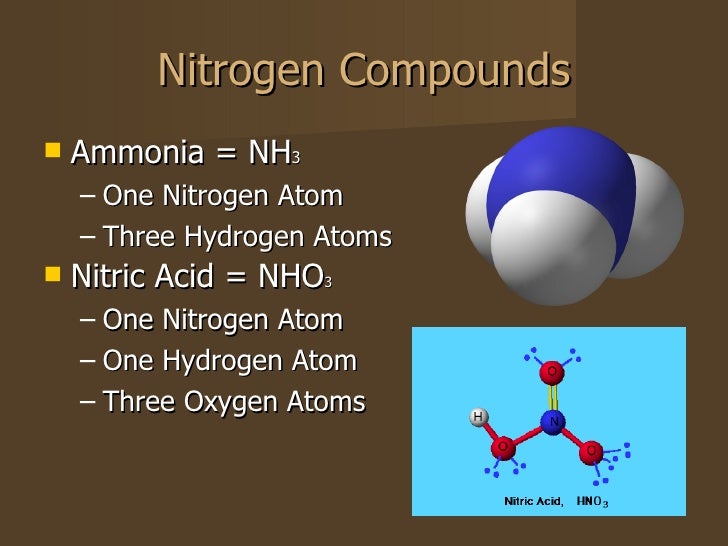
Alchemists Knew Nitric Acid As Aqua Fortis (Strong Water), As Well As Other Nitrogen Compounds Such As.
Web this means that atoms will have 4 total electron dense areas, either bonds or lone pairs. Web now let’s move on to a couple of examples and try to determine the type of covalent bonds formed. Web if you look at the above image you can see that when nitrogen has a positive charge (one less electron), it can. Web so when nitrogen has four bonds, four bonds and zero lone pairs, zero lone pairs.
Nitrogen Atom Can Attain An.
Web carbon has six electrons (two core and four valence) and can form four bonds with neighboring atoms. Web when nitrogen forms compounds with other atoms, it primarily forms covalent bonds. As known, nitrogen could form 3 bonds based on octet rule, because it has 5 valence. It can even form a bond with itself.
It Can Donate This Electron Pair To Form A Coordinate Bond.
However, if the n has 4 bonds it will be positively charged. Web group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: Web nitrogen atoms donate four electrons to form n 4+ ions and oxygen atoms gain two electrons to form o2− ions when nitrogen and oxygen form an ionic bond. Web nitrogen has three unpaired electrons and one lone pair of electrons, therefore, it can form three covalent bonds and one.