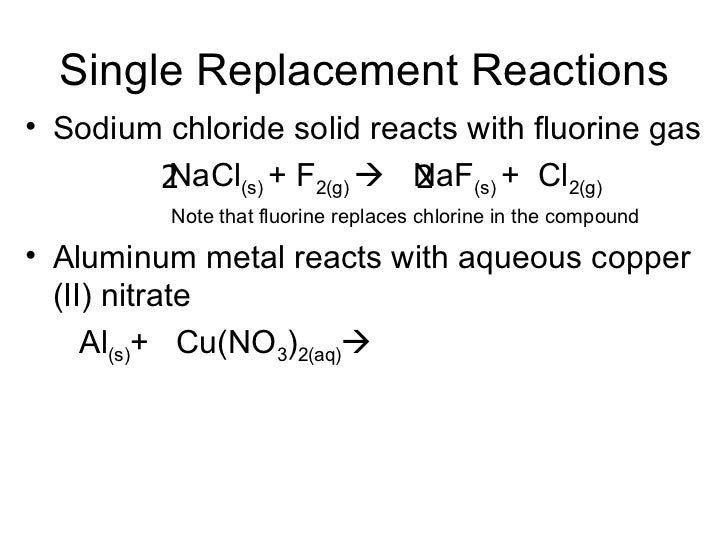
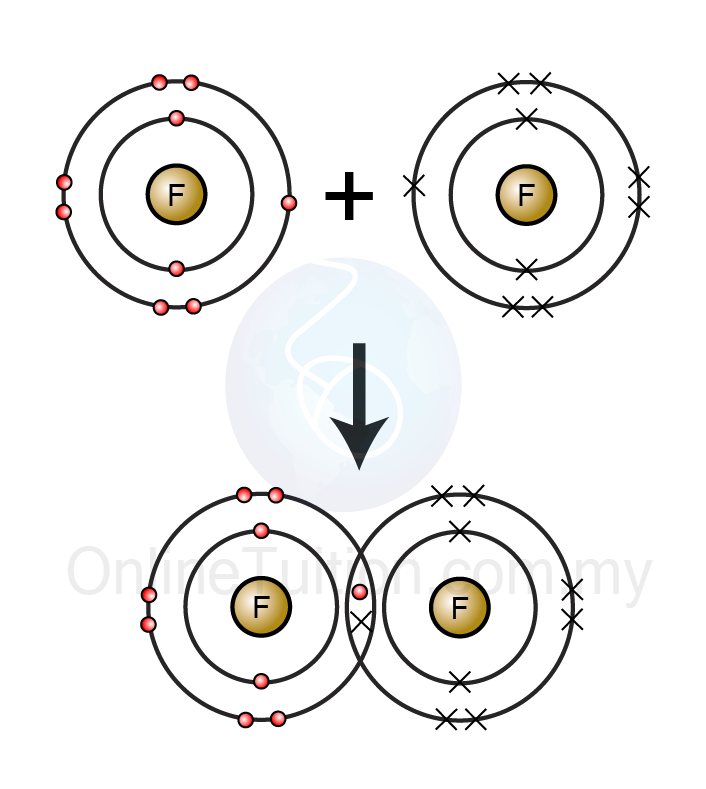
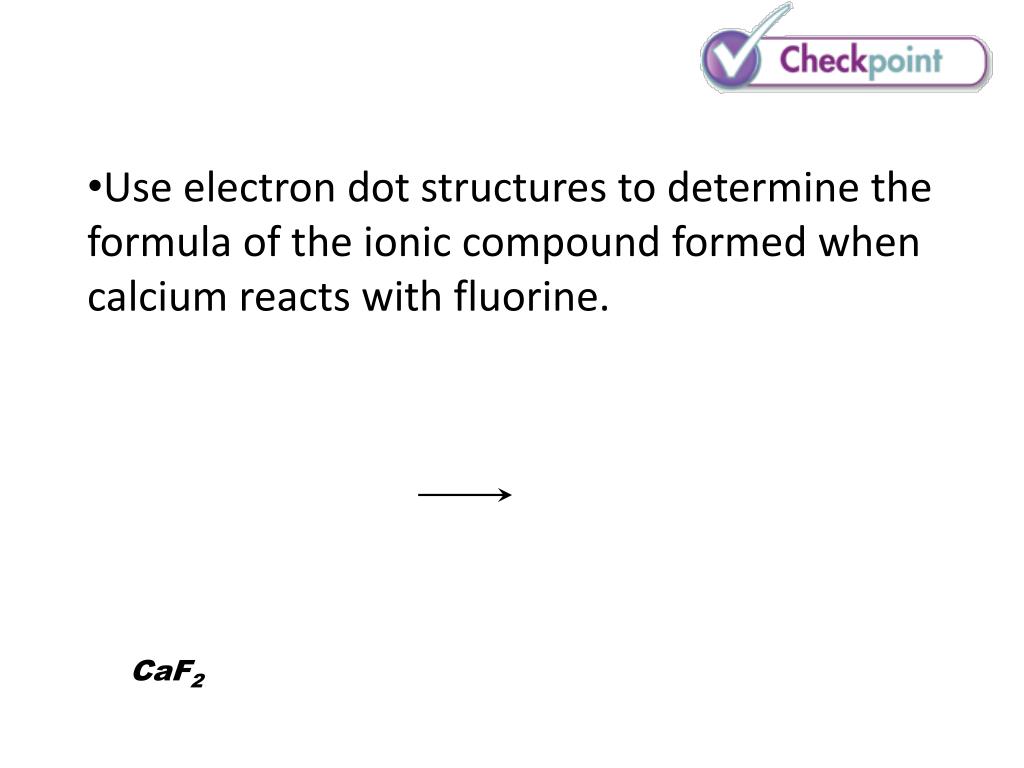
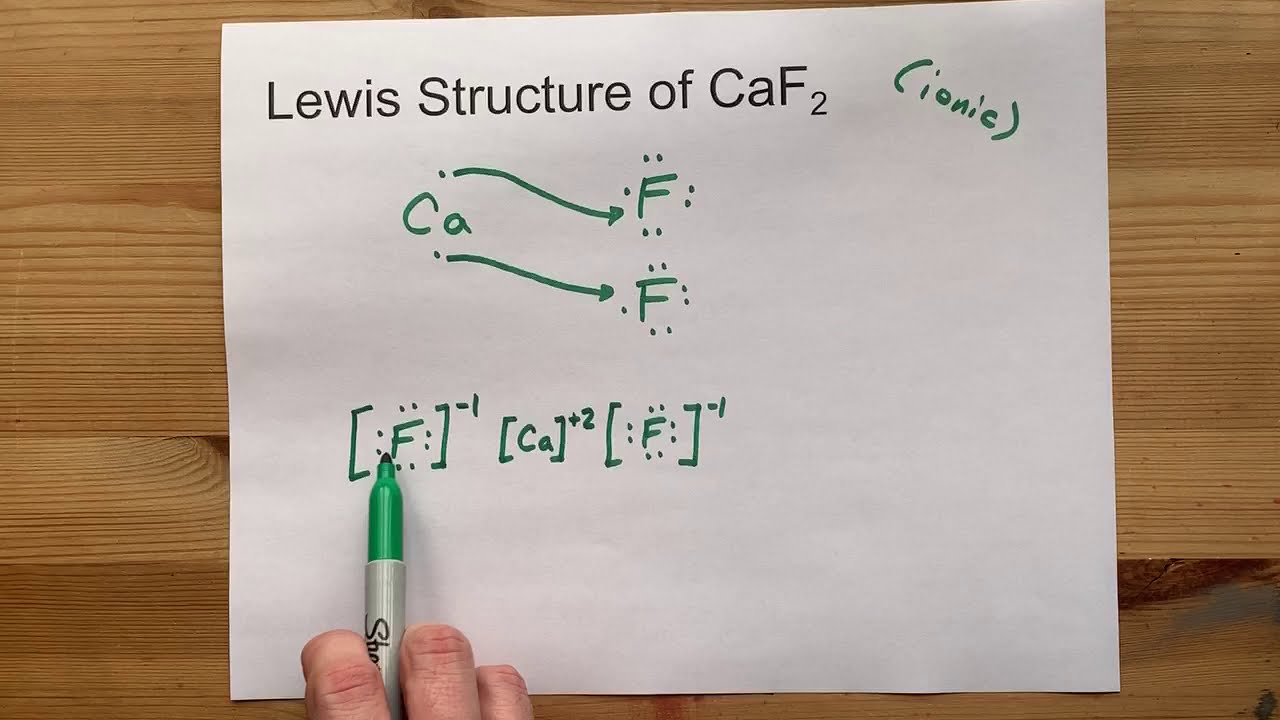
Calcium Reacts With Fluorine To Form - Web calcium reacts with fluorine to form what? It is one calcium atom bonded to two fluoride atoms. Ca + f₂ → caf₂. Calcium reacts with fluorine to form the compound $\mathrm{caf}_{2}$. This is an example of an ionic bond forming. Web this problem has been solved! Web many of the difluorides adopt the fluorite structure, named after calcium fluoride (and also adopted by several metal dioxides. Web when calcium reacts with fluorine to form an ionic compound, each metal atom loses _ electron(s) and each. Web when calcium reacts with fluorine to produce calcium fluoride, calcium is oxidized and fluorine is reduced. Web 5 lithium reacts with fluorine to form the compound lithium fluoride.
Chemical Reactions
What volume of fluorine gas (in. This is an example of an ionic bond forming. Web fluoride is the ionic form of the element fluorine, negatively charged and combines with positive ions (e.g., calcium or. Web calcium + flourine → calcium flouride. When the reaction is balanced, what.
science chemistry precipitation reaction calcium fluoride Fundamental
Ca + f₂ → caf₂. This equation shows that 1 mole of. Web answer 1 calcium fluoride is produced. Web the balanced equation of the chemical reaction is as follows: Web calcium + flourine → calcium flouride.
Calcium Fluoride Crystal Structure YouTube
The calcium atom receives one. Web this problem has been solved! Web calcium fluoride (caf2) is an insoluble ionic compound composed of ca 2+ and f − ions. Ca + f₂ → caf₂. Web many of the difluorides adopt the fluorite structure, named after calcium fluoride (and also adopted by several metal dioxides.
science chemistry precipitation reaction calcium fluoride Fundamental
Web expert answer top expert 500+ questions answered transcribed image text: Web this problem has been solved! This is an example of an ionic bond forming. Web problem 122 easy difficulty. Web 5 lithium reacts with fluorine to form the compound lithium fluoride.
Chemical Bond SPM Chemistry
What volume of fluorine gas (in. Web this problem has been solved! The calcium atom receives one. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web many of the difluorides adopt the fluorite structure, named after calcium fluoride (and also adopted by several metal dioxides.
PPT Chapter 7 PowerPoint Presentation, free download ID2380065
Web calcium reacts with fluorine to form what? Ca + f₂ → caf₂. Web when calcium reacts with fluorine to produce calcium fluoride, calcium is oxidized and fluorine is reduced. Web calcium metal reacts with fluorine gas to form solid calcium fluoride. Web answer 1 calcium fluoride is produced.
SOLVEDCalcium reacts with fluorine to form the compound CaF2. In the
What volume of fluorine gas (in. Web the balanced equation of the chemical reaction is as follows: This is an example of an ionic bond forming. Web expert answer top expert 500+ questions answered transcribed image text: Web calcium reacts with fluorine to form what?
Chemical Reactions
We are given fluorine gas which is held in contact with the calcium metal and produces calcium. Ca + f₂ → caf₂. Web many of the difluorides adopt the fluorite structure, named after calcium fluoride (and also adopted by several metal dioxides. Web this problem has been solved! Web calcium fluoride (caf2) is an insoluble ionic compound composed of ca.
Calcium Fluoride And Sulfuric Acid Make Calcium Sulfate And Hydrogen
Web calcium reacts with fluorine to form what? What volume of fluorine gas (in. Web calcium fluoride (caf2) is an insoluble ionic compound composed of ca 2+ and f − ions. When the reaction is balanced, what. Web when calcium reacts with fluorine to produce calcium fluoride, calcium is oxidized and fluorine is reduced.
Draw the Lewis Structure of CaF2 (calcium fluoride) YouTube
Calcium reacts with fluorine to form the compound $\mathrm{caf}_{2}$. Web fluorine gas reacts with solid calcium bromide to form calcium fluoride and liquid bromine. Web when calcium reacts with fluorine to form an ionic compound, each metal atom loses _ electron(s) and each. Which statement about this reaction is correct? Web 5 lithium reacts with fluorine to form the compound.
Web this problem has been solved! Web when calcium reacts with fluorine to form an ionic compound, each metal atom loses _ electron(s) and each. Calcium fluoride which is the correct formula for the ionic compound. We are given fluorine gas which is held in contact with the calcium metal and produces calcium. Web calcium fluoride (caf2) is an insoluble ionic compound composed of ca 2+ and f − ions. Ca + f_2 → caf_2. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. When the reaction is balanced, what. Web calcium + flourine → calcium flouride. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web expert answer top expert 500+ questions answered transcribed image text: Web the balanced equation of the chemical reaction is as follows: Web calcium metal reacts with fluorine gas to form solid calcium fluoride. What volume of fluorine gas (in. Ca + f₂ → caf₂. It occurs naturally as the mineral “fluorite”. Web fluoride is the ionic form of the element fluorine, negatively charged and combines with positive ions (e.g., calcium or. Web fluorine gas reacts with solid calcium bromide to form calcium fluoride and liquid bromine. Which statement about this reaction is correct? It is one calcium atom bonded to two fluoride atoms.
It Is One Calcium Atom Bonded To Two Fluoride Atoms.
What volume of fluorine gas (in. Web problem 122 easy difficulty. This equation shows that 1 mole of. Web fluoride is the ionic form of the element fluorine, negatively charged and combines with positive ions (e.g., calcium or.
Web Answer 1 Calcium Fluoride Is Produced.
Web expert answer top expert 500+ questions answered transcribed image text: Web calcium metal reacts with fluorine gas to form solid calcium fluoride. Web fluorine gas reacts with solid calcium bromide to form calcium fluoride and liquid bromine. Calcium reacts with fluorine to form the compound $\mathrm{caf}_{2}$.
The Calcium Atom Receives One.
Web this problem has been solved! Web when calcium reacts with fluorine to produce calcium fluoride, calcium is oxidized and fluorine is reduced. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web when calcium reacts with fluorine to produce calcium fluoride, calcium is oxidized and fluorine is reduced.
Web Calcium Reacts With Fluorine To Form What?
This is an example of an ionic bond forming. When the reaction is balanced, what. Web the balanced equation of the chemical reaction is as follows: Web it was found that, in reaction between fluorine and carbon dioxide, fluorine anions react with co 2 to form (co 2 f) −.