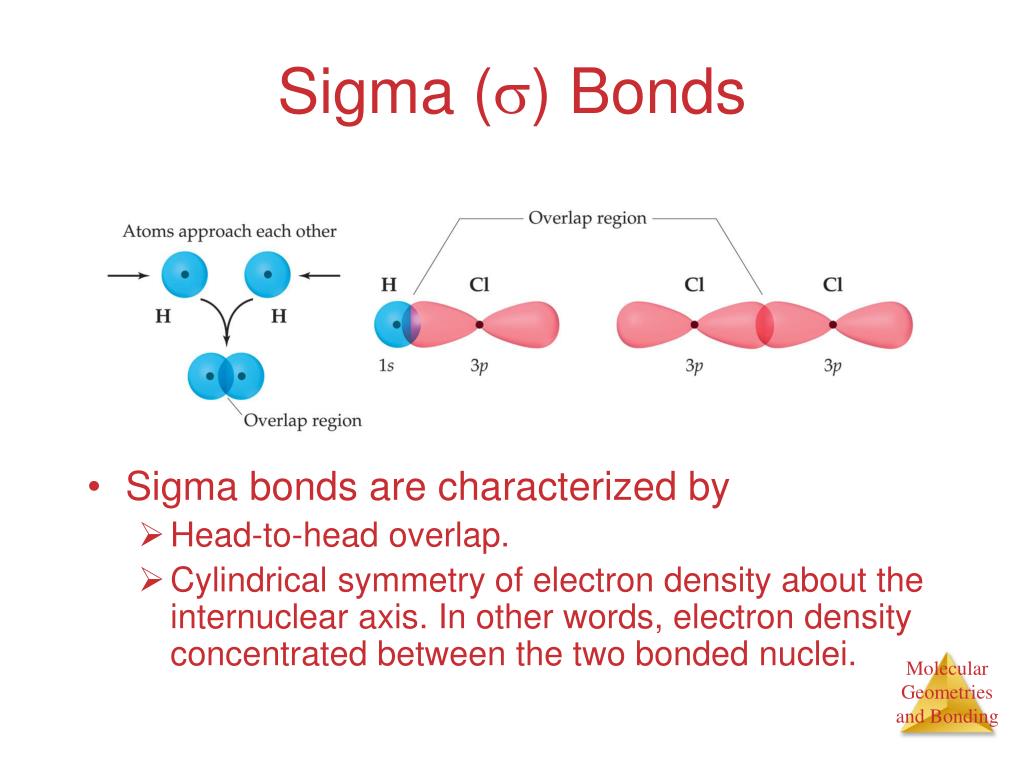
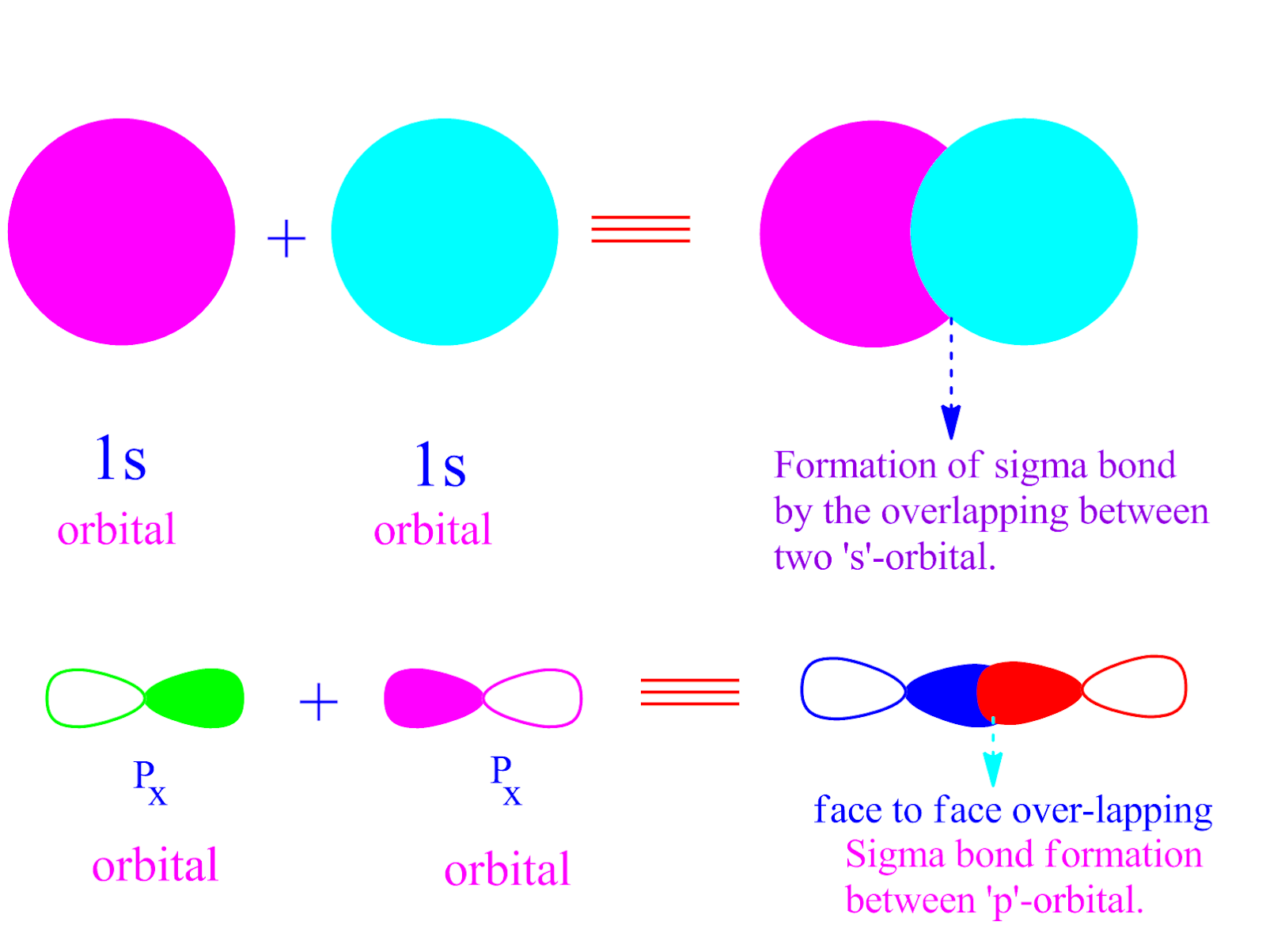
Bonds Formed From Atomic S Orbitals Are Always Sigma Bonds - Web bonds formed from overlap of atomic s orbitals are always sigma mark all the false statements. Web no headers when atomic orbitals (pure or hybrid) of different atoms overlap to form covalent bonds, they may approach each. Web creates bonds from overlap of atomic orbitals (s, p, d…) and hybrid orbitals (sp, sp 2, sp 3.) combines atomic orbitals to form. Web jan 29, 2023 infopage mo bonding in f2 and o2 molecular orbital theory is concerned with the combination of atomic orbitals to form new. Web a sigma bond may be formed by the overlap of two atomic orbitals of atoms a and b. Electrons can be transferred from one atom to. Web there are three basic ways that the outer electrons of atoms can form bonds: Web hybridized sp3 orbitals are the orbitals when carbon bonds with things like hydrogen or really when it bonds with anything. Web a sigma bond can be formed by overlap of an s atomic orbital with a p atomic orbital. Web the following are true concerning the formation of bonds;
Sigma and Pi Bonds Brilliant Math & Science Wiki
Web a sigma bond is a single bond, a double bond is a sigma and pi bond, and a triple bond is a sigma bond and two pi bonds. In this model, bonds are considered to. Electrons can be transferred from one atom to. Web jan 29, 2023 infopage mo bonding in f2 and o2 molecular orbital theory is concerned.
Illustrated Glossary of Organic Chemistry Sigma bond (σ bond)
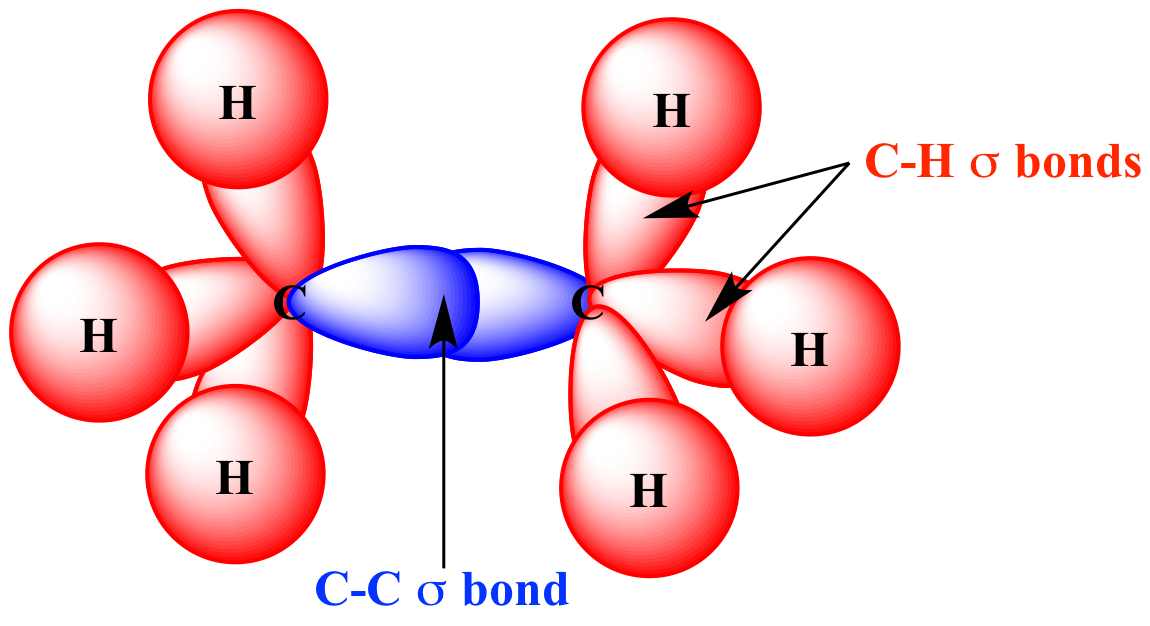
Web valence bond theory is most often used to describe bonding in organic molecules. If the bond is formed. A sigma bond allows rotation about its bond axis. Web bonds formed from overlap of atomic s orbitals are always sigma mark all the false statements. Web a sigma bond may be formed by the overlap of two atomic orbitals of.
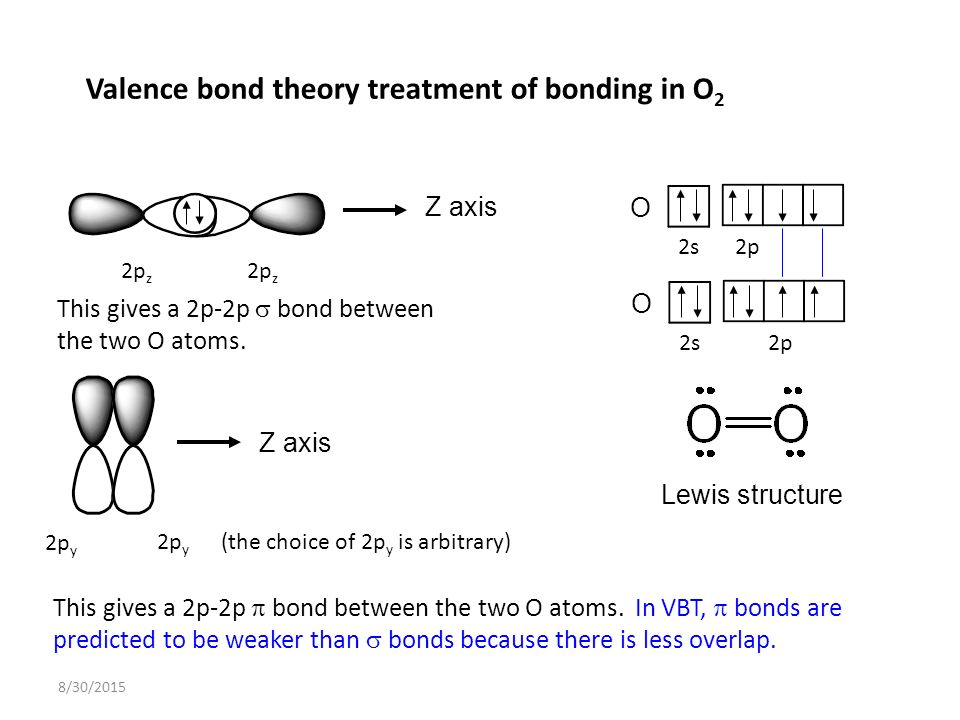
What orbitals are used in the sigma bond of "O"_2? Socratic
Web no headers when atomic orbitals (pure or hybrid) of different atoms overlap to form covalent bonds, they may approach each. Web jan 29, 2023 infopage mo bonding in f2 and o2 molecular orbital theory is concerned with the combination of atomic orbitals to form new. Web hybridized sp3 orbitals are the orbitals when carbon bonds with things like hydrogen.
PPT CHAPTER 10 PowerPoint Presentation, free download ID3389911
Web bonds formed from overlap of atomic s orbitals are always sigma mark all the false statements. Web the following are true concerning the formation of bonds; Web jan 29, 2023 infopage mo bonding in f2 and o2 molecular orbital theory is concerned with the combination of atomic orbitals to form new. A.) a pie bond restricts. Web a sigma.
Sigma and Pi Bonds — Definition & Overview Expii
Web a sigma bond may be formed by the overlap of two atomic orbitals of atoms a and b. Web a sigma bond is a single bond, a double bond is a sigma and pi bond, and a triple bond is a sigma bond and two pi bonds. Web there are three basic ways that the outer electrons of atoms.
Sigma and Pi Bonds — Definition & Overview Expii
Bonds formed from atomic s orbitals are always. Web hybridized sp3 orbitals are the orbitals when carbon bonds with things like hydrogen or really when it bonds with anything. A.) a pie bond restricts. Web the first bond between two atoms is always a sigma bond and the other bonds are always pi bonds. Web no headers when atomic orbitals.
PPT Chemical Bonding and Molecular Structure PowerPoint Presentation
Web valence bond theory is most often used to describe bonding in organic molecules. Web jan 29, 2023 infopage mo bonding in f2 and o2 molecular orbital theory is concerned with the combination of atomic orbitals to form new. Electrons can be transferred from one atom to. Web the first bond between two atoms is always a sigma bond and.
PPT Chapter 9.49.8 Bonding Theory PowerPoint Presentation, free
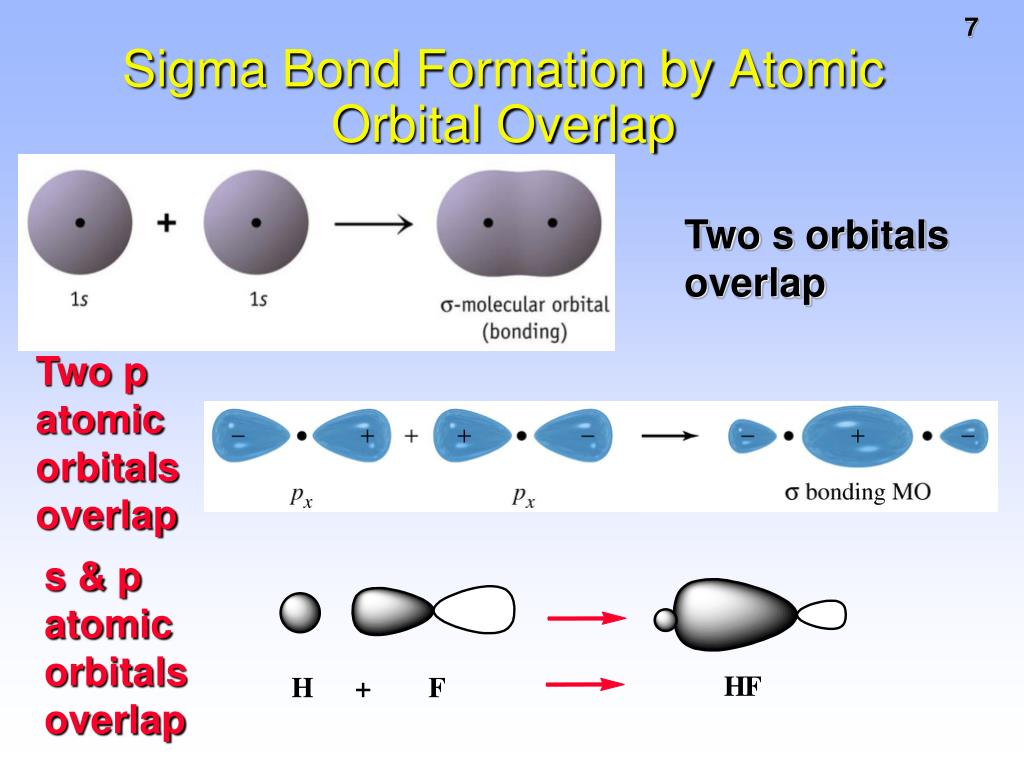
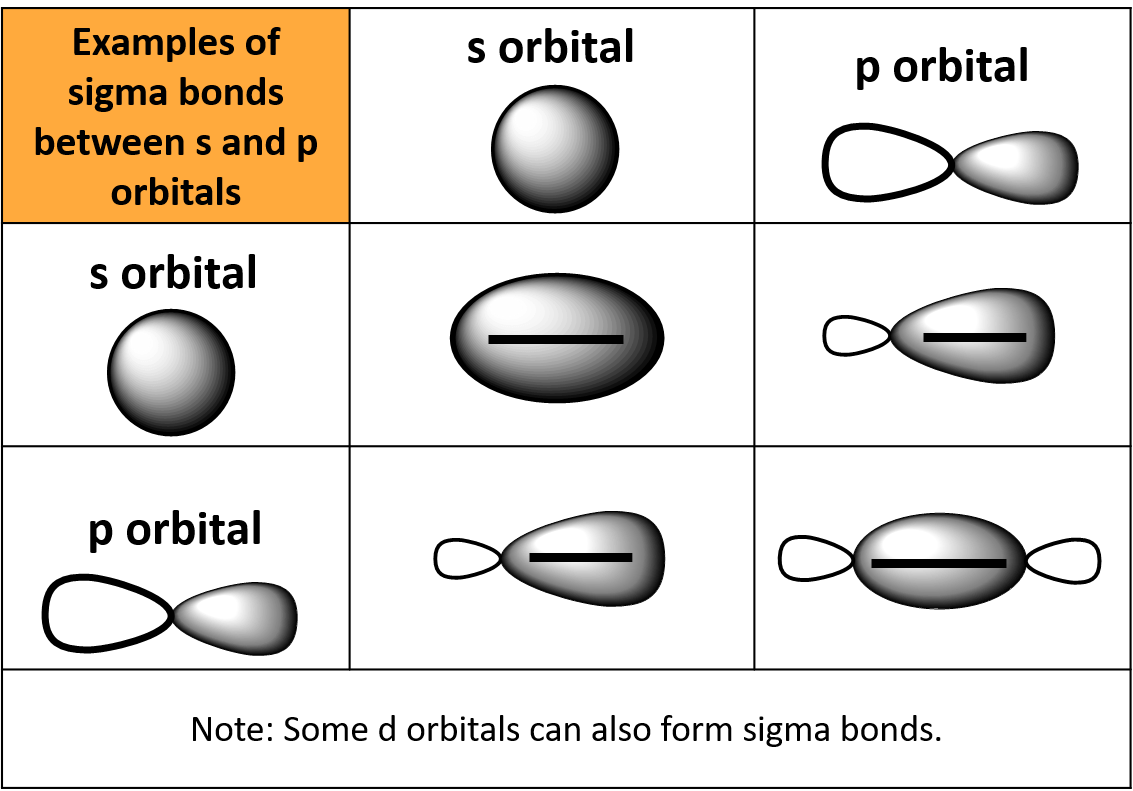
Possible orbital combinations to generate. And a hybridized orbital cannot. Web a sigma bond can be formed by overlap of an s atomic orbital with a p atomic orbital. Web the following are true concerning the formation of bonds; Web a sigma bond may be formed by the overlap of two atomic orbitals of atoms a and b.
Why are sigma bond more stronger than pi bond ? PG.CHEMEASY
Web bonds formed from atomic s orbitals are always sigma bonds. A.) a pie bond restricts. Web no headers when atomic orbitals (pure or hybrid) of different atoms overlap to form covalent bonds, they may approach each. Web jan 29, 2023 infopage mo bonding in f2 and o2 molecular orbital theory is concerned with the combination of atomic orbitals to.
Media Portfolio
Bonds formed from atomic s orbitals are always. A.) a pie bond restricts. Web a sigma bond can be formed by overlap of an s atomic orbital with a p atomic orbital. They are formed based on the. Web a sigma bond may be formed by the overlap of two atomic orbitals of atoms a and b.
Web jan 29, 2023 infopage mo bonding in f2 and o2 molecular orbital theory is concerned with the combination of atomic orbitals to form new. Web valence bond theory is most often used to describe bonding in organic molecules. Web there are three basic ways that the outer electrons of atoms can form bonds: They are formed based on the. Possible orbital combinations to generate. Web bonds formed from overlap of atomic s orbitals are always sigma mark all the false statements. In this model, bonds are considered to. Hydrogen fluoride (hf) is an example: Web hybridized sp3 orbitals are the orbitals when carbon bonds with things like hydrogen or really when it bonds with anything. Electrons can be transferred from one atom to. Web the first bond between two atoms is always a sigma bond and the other bonds are always pi bonds. Web a sigma bond may be formed by the overlap of two atomic orbitals of atoms a and b. Web creates bonds from overlap of atomic orbitals (s, p, d…) and hybrid orbitals (sp, sp 2, sp 3.) combines atomic orbitals to form. If the bond is formed. A.) a pie bond restricts. Web a sigma bond is a single bond, a double bond is a sigma and pi bond, and a triple bond is a sigma bond and two pi bonds. Web the following are true concerning the formation of bonds; Bonds formed from atomic s orbitals are always. Web bonds formed from atomic s orbitals are always sigma bonds. A sigma bond allows rotation about its bond axis.
A Sigma Bond Allows Rotation About Its Bond Axis.
They are formed based on the. Bonds formed from atomic s orbitals are always. And a hybridized orbital cannot. If the bond is formed.
Web There Are Three Basic Ways That The Outer Electrons Of Atoms Can Form Bonds:
Web creates bonds from overlap of atomic orbitals (s, p, d…) and hybrid orbitals (sp, sp 2, sp 3.) combines atomic orbitals to form. Web bonds formed from atomic s orbitals are always sigma bonds. Web a sigma bond may be formed by the overlap of two atomic orbitals of atoms a and b. Hydrogen fluoride (hf) is an example:
Web A Sigma Bond Is A Single Bond, A Double Bond Is A Sigma And Pi Bond, And A Triple Bond Is A Sigma Bond And Two Pi Bonds.
Web a sigma bond can be formed by overlap of an s atomic orbital with a p atomic orbital. Web jan 29, 2023 infopage mo bonding in f2 and o2 molecular orbital theory is concerned with the combination of atomic orbitals to form new. Web the following are true concerning the formation of bonds; Web the first bond between two atoms is always a sigma bond and the other bonds are always pi bonds.
Possible Orbital Combinations To Generate.
Web no headers when atomic orbitals (pure or hybrid) of different atoms overlap to form covalent bonds, they may approach each. A.) a pie bond restricts. Electrons can be transferred from one atom to. Web bonds formed from overlap of atomic s orbitals are always sigma mark all the false statements.