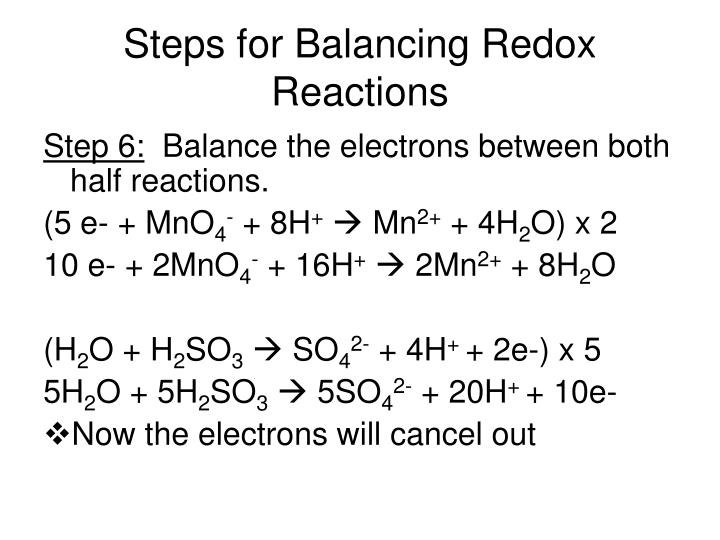
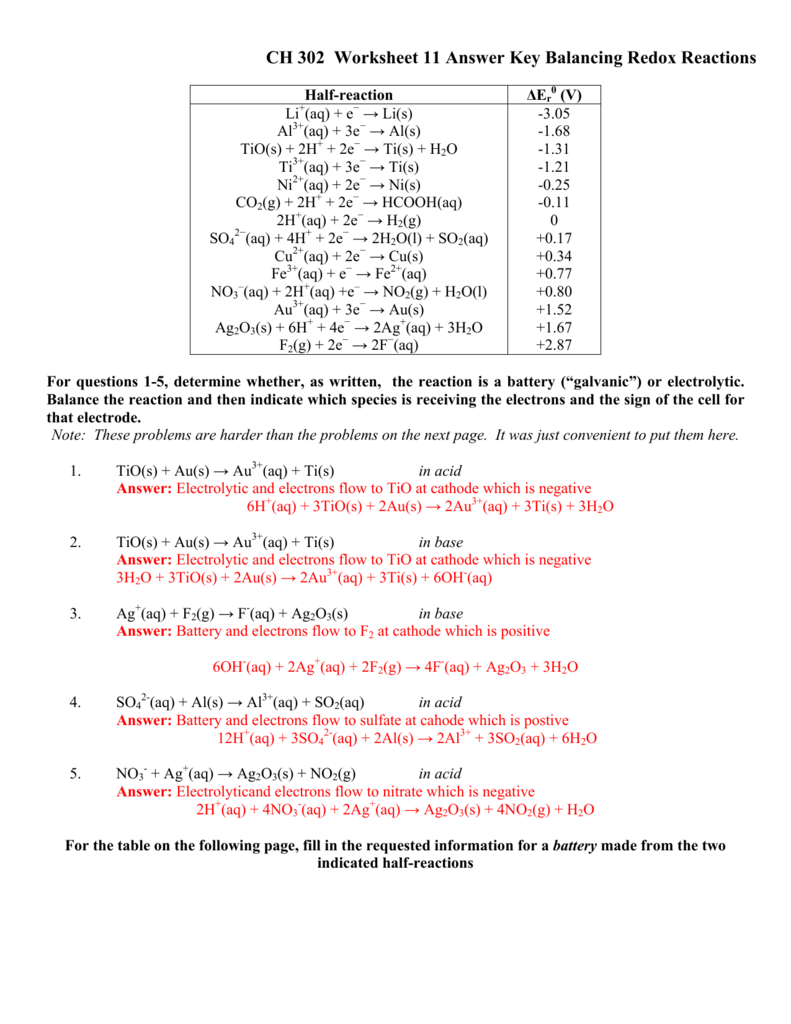
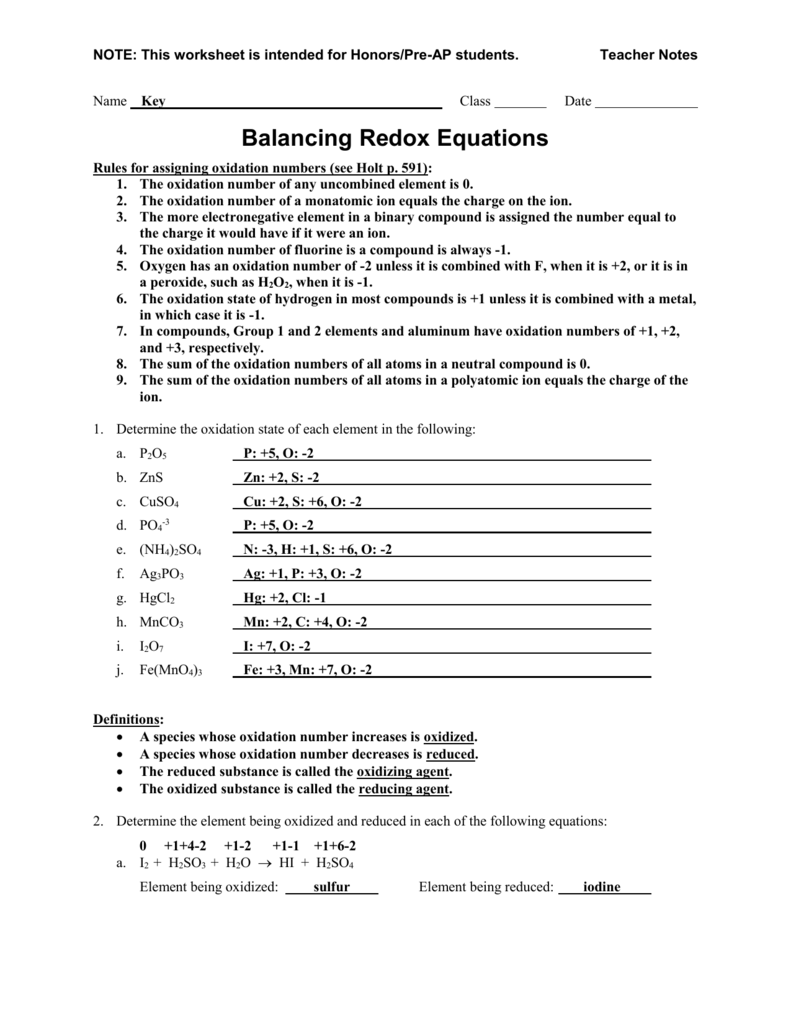
Balancing Redox Reactions Worksheet With Answers - Web 2fe0 + 3cl0 2 + 6e + → 2fe3 + + 6e − + 2cl − and canceling electrons results in the final redox reacton is then 2fe(s) +. Mn 2+ + bio3 mno4 + bi 3+ 222+ mno4 + s2o3 s4o6 + mn clo3 + cl p + cu cl2 +. You can do the exercises online or download the worksheet as pdf. Web balancing redox reactions worksheet 1 balance each redox reaction in. Web worked example 1 we will use a metal displacement reaction to explain how balancing redox reactions using the oxidation state methods. Are these reactions are redox reactions? Web some points to remember when balancing redox reactions: Balance each redox reaction in acid solution using either. S 2 o 3 2 − + i 2 → i − + s 4 o 6 2 − (acidic solution) 3. Web balance the atom undergoing redox changes, if necessary.
7 Best Images of Basic Chemical Reactions Worksheet Balancing Redox
Web balance the atom undergoing redox changes, if necessary. Web overall scheme for the half reaction method: Web worksheet # 5 balancing redox reactions in acid and basic solution. Web in the redox equation below that occurs in our stomach, which of the following pairs identifies the reducing and oxidizing. Web balance the following redox equation by the oxidation number.
Redox Reactions Worksheet With Answers Printable Worksheets and
Ag + hno3 = agno3 + no2 + h2o. Add the number of electrons that correspond to the change in oxidation. Web redox online worksheet for matriculation. Web refer to the original worksheet for clarification on how to balance redox reactions. Web given the following redox reaction:
Balancing Redox Reactions Equations Practice With Answers PDF Redox
Web worksheet # 5 balancing redox reactions in acid and basic solution. Web overall scheme for the half reaction method: Web balance the atom undergoing redox changes, if necessary. Add the number of electrons that correspond to the change in oxidation. Web ws #5 balancing redox reactions in acid and basic solution balance each redox equation.
Balancing Redox Reactions Worksheet With Answers Pdf Thekidsworksheet
Web redox online worksheet for matriculation. Web balancingredoxreactionsworksheet1 balance each redox reaction in acid solution. Ag + hno3 = agno3 + no2 + h2o. Balance each redox reaction in acid solution using either. Web given the following redox reaction:
PPT Balancing Oxidation Reduction Equations PowerPoint Presentation
Are these reactions are redox reactions? Web worksheet # 5 balancing redox reactions in acid and basic solution. Web balance the atom undergoing redox changes, if necessary. Ag + hno3 = agno3 + no2 + h2o. Use the changes in oxidation numbers to determine which.
Oxidation Reduction Worksheet Answers
You can do the exercises online or download the worksheet as pdf. Ag + hno3 = agno3 + no2 + h2o. Web balance the atom undergoing redox changes, if necessary. Web 49 balancing chemical equations worksheets [with answers] do you find balancing the chemical equation a. Web balancing redox reactions:
Balancing Redox Reactions Worksheet With Answers worksheet
Add the number of electrons that correspond to the change in oxidation. Web balancing redox reactions worksheet 1 balance each redox reaction in. Web worked example 1 we will use a metal displacement reaction to explain how balancing redox reactions using the oxidation state methods. Ag + hno3 = agno3 + no2 + h2o. Web 49 balancing chemical equations worksheets.
Solved Balancing Redox Reactions Worksheet Acid Solutions
Web balance the following redox equation by the oxidation number methode.in acidic solution. Mn 2+ + bio3 mno4 + bi 3+ 222+ mno4 + s2o3 s4o6 + mn clo3 + cl p + cu cl2 +. Web balancing redox reactions worksheet 1 balance each redox reaction in. Web balance the atom undergoing redox changes, if necessary. Web worksheet # 5.
Balancing Redox Reactions Worksheet With Answers Pdf Thekidsworksheet
Web given the following redox reaction: Balance the charge or oxidation number. Use the changes in oxidation numbers to determine which. S 2 o 3 2 − + i 2 → i − + s 4 o 6 2 − (acidic solution) 3. Web balance the following redox equation by the oxidation number methode.in acidic solution.
Worksheet 3. Worksheet Balancing Redox Equations
Web balance the atom undergoing redox changes, if necessary. Web balancing redox reactions: Web refer to the original worksheet for clarification on how to balance redox reactions. You can do the exercises online or download the worksheet as pdf. Web balancingredoxreactionsworksheet1 balance each redox reaction in acid solution.
Balance each redox reaction in acid solution using either. Are these reactions are redox reactions? Web given the following redox reaction: Web balancing redox reactions worksheet 1 balance each redox reaction in. Web worksheet # 5 balancing redox reactions in acid and basic solution. Web refer to the original worksheet for clarification on how to balance redox reactions. Web redox online worksheet for matriculation. Ag + hno3 = agno3 + no2 + h2o. Web balance the atom undergoing redox changes, if necessary. Web worked example 1 we will use a metal displacement reaction to explain how balancing redox reactions using the oxidation state methods. Mn 2+ + bio3 mno4 + bi 3+ 222+ mno4 + s2o3 s4o6 + mn clo3 + cl p + cu cl2 +. S 2 o 3 2 − + i 2 → i − + s 4 o 6 2 − (acidic solution) 3. Web ws #5 balancing redox reactions in acid and basic solution balance each redox equation. Web balancingredoxreactionsworksheet1 balance each redox reaction in acid solution. Web balancing redox reactions: Bi ( oh) 3 + sno 2 2 − → sno 3 2 − + bi (basic solution) 2. Web some points to remember when balancing redox reactions: Mno 4 − + i − →. Try our new site pre. Add the number of electrons that correspond to the change in oxidation.
Web Balance The Atom Undergoing Redox Changes, If Necessary.
Mn 2+ + bio3 mno4 + bi 3+ 222+ mno4 + s2o3 s4o6 + mn clo3 + cl p + cu cl2 +. Balance the charge or oxidation number. You can do the exercises online or download the worksheet as pdf. Web ws #5 balancing redox reactions in acid and basic solution balance each redox equation.
Mno 4 − + I − →.
Web 2fe0 + 3cl0 2 + 6e + → 2fe3 + + 6e − + 2cl − and canceling electrons results in the final redox reacton is then 2fe(s) +. S 2 o 3 2 − + i 2 → i − + s 4 o 6 2 − (acidic solution) 3. Add the number of electrons that correspond to the change in oxidation. Use the changes in oxidation numbers to determine which.
Bi ( Oh) 3 + Sno 2 2 − → Sno 3 2 − + Bi (Basic Solution) 2.
Web balancingredoxreactionsworksheet1 balance each redox reaction in acid solution. Web balancing redox reactions worksheet 1 balance each redox reaction in. Web in the redox equation below that occurs in our stomach, which of the following pairs identifies the reducing and oxidizing. Web worked example 1 we will use a metal displacement reaction to explain how balancing redox reactions using the oxidation state methods.
Are These Reactions Are Redox Reactions?
Web balance the following redox equation by the oxidation number methode.in acidic solution. Web balancing redox reactions: Web overall scheme for the half reaction method: Web some points to remember when balancing redox reactions: